Real-world treatment and retreatment patterns and outcomes in patients with advanced or metastatic non-small cell lung cancer following nivolumab monotherapy in second line or later in France: an I-O Optimise analysis
- PMID: 40052124
- PMCID: PMC11883363
- DOI: 10.3389/fonc.2025.1526931
Real-world treatment and retreatment patterns and outcomes in patients with advanced or metastatic non-small cell lung cancer following nivolumab monotherapy in second line or later in France: an I-O Optimise analysis
Abstract
Introduction: This study describes treatment and retreatment patterns and outcomes in patients in France following nivolumab as a second-line or later (2L+) treatment in locally advanced or metastatic non-small cell lung cancer (LAM NSCLC).
Materials and methods: This analysis included adults with tumor, node, metastasis stage IIIB-IV NSCLC (as defined in the 7th or 8th edition American Joint Committee on Cancer/Union for International Cancer Control) treated with nivolumab monotherapy in 2L+ using data from the retrospective Epidemiological-Strategy and Medical Economics Lung Cancer database. The inclusion period was from January 1, 2015, to September 30, 2020, with a follow-up until September 30, 2021. Analyses were stratified according to the duration of index nivolumab treatment and tumor programmed death ligand 1 expression levels.
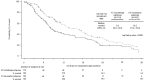
Results: In total, the study included 4,001 patients (68% male; mean age [standard deviation] at index date, 63.6 [9.7] years) with a median follow-up of 34.3 months. The median nivolumab duration was 2.5 months (interquartile range, 1.4-6.3). The median overall survival (OS) from nivolumab initiation was 10.2 months (95% confidence interval [CI], 9.6-10.8). The median real-world progression-free survival and time to treatment discontinuation or death (95% CI) were 2.2 (2.1-2.3) and 2.7 (2.5-2.8) months, respectively. In total, 2,985 (74.6%) patients discontinued index nivolumab treatment: 226 (7.6% of discontinuers) received a further immune checkpoint inhibitor (ICI; 12.3% of discontinuers receiving further systemic treatment), and 1,604 (53.7%) received chemotherapy and/or targeted therapy. The proportion of ICI-retreated patients was the highest among those with the longest index treatment duration (15.8% among discontinuers receiving ≥26 weeks' index nivolumab). The median OS from retreatment was longer in the resumption (ICI restart without another therapy for ≥6 weeks) compared with the rechallenge (ICI restart following non-ICI therapy) patient subgroup.
Conclusion: Few patients with LAM NSCLC in France received ICI retreatment following index nivolumab discontinuation, but the proportion increased with a longer duration of index nivolumab.
Keywords: NSCLC; PD-L1 expression; immunotherapy; real-world; rechallenge; retreatment.
Copyright © 2025 Justeau, Chouaid, Debieuvre, Audigier-Valette, Quantin, Léna, Bosquet, Girard, Schoemaker, Mella, Pinto Correia, Rault, Daumont, Penrod, Lee and Pérol.
Conflict of interest statement
GJ reports support for attending meetings and/or travel from Sanofi. CC reports consulting fees and support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche, Sanofi Aventis, Bristol Myers Squibb, Merck Sharp & Dohme, Eli Lilly, Novartis, Pfizer, Takeda, Bayer, and Amgen. CA-V reports payment for participation on advisory boards for Roche, Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Sanofi, Janssen, Amgen, and Biodena and as an invited speaker for Pfizer; along with non-financial interests through advisory role for IFCT scientific committee; GFPC Principal Investigator; Member of Board of Directors, Editorial Committee, Edimark; Lettre du cancérologue; and Actualités en oncologie thoracique and President of 3C Var-Ouest. XQ reports participation on a data safety monitoring board and leadership or fiduciary role in other boards, society, committees, or advocacy groups, paid or unpaid for Bristol Myers Squibb. HL reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb, Pfizer, Roche, Lilly, Daiichi-Sankyo, Takeda, Amgen; and support for attending meetings and/or travel from Roche, Takeda, Pfizer, and Amgen. LB is an employee of Unicancer. NG reports receipt of research grants/support from AbbVie, Amgen, AstraZeneca, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Gilead, Hoffmann-La Roche, Janssen, LeoPharma, Lilly, Merk Serono, Merck Sharp & Dohme, Novartis, Sanofi, and Sivan; consultative services for AbbVie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb, Daiichi-Sankyo, Gilead, Ipsen, Hoffmann-La Roche, Janssen, LeoPharma, Lilly, Merck Sharp & Dohme, Mirati, Novartis, Pfizer, Pierre Fabre, Sanofi, and Takeda; participation on a data safety monitoring board for Hoffmann-La Roche; and employment of a family member with AstraZeneca. MS, MM, and BPC are employees of IQVIA. MD, JP, and AL are employees of Bristol Myers Squibb and report stock ownership. MP reports consulting fees from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Roche, Daiichi Sankyo, Janssen, Ipsen, Esai, GlaxoSmithKline, Eli Lilly, Pfizer, Takeda, and Novocure; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, AnHeart Therapeutics, Sanofi, Pfizer, Takeda, and Janssen; payment for expert testimony from Bristol Myers Squibb, AstraZeneca, Roche, and Janssen; support for attending meetings and/or travel from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Roche, Pfizer, and Takeda; and participation on a data safety monitoring board or advisory board for Roche and Pharmamar. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Bristol Myers Squibb. The funder was involved in the study design and decision to publish, and funded the data collection and analysis and the preparation of the manuscript.
Figures


References
-
- GLOBOCAN . Global Cancer Observatory (2020) (Accessed January 23, 2024).
-
- Debieuvre D, Molinier O, Falchero L, Locher C, Templement-Grangerat D, Meyer N, et al. . Lung cancer trends and tumor characteristic changes over 20 years (2000-2020): Results of three French consecutive nationwide prospective cohorts' studies. Lancet Reg Health Eur. (2022) 22:100492. doi: 10.1016/j.lanepe.2022.100492 - DOI - PMC - PubMed
-
- European Society for Medical Oncology, ESMO . Guidelines for Metastatic NSCLC (2020). Available online at: https://interactiveguidelines.esmo.org/esmo-web-app/toc/index.php?subjec... (Accessed January 23, 2024).
LinkOut - more resources
Full Text Sources
Research Materials

