Therapeutic Treatment With OLX-07010 Inhibited Tau Aggregation and Ameliorated Motor Deficits in an Aged Mouse Model of Tauopathy
- PMID: 40052227
- PMCID: PMC11886763
- DOI: 10.1111/jnc.70025
Therapeutic Treatment With OLX-07010 Inhibited Tau Aggregation and Ameliorated Motor Deficits in an Aged Mouse Model of Tauopathy
Abstract
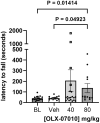
Targeting tau protein is a strategy for the development of disease-modifying therapeutics for Alzheimer's disease (AD) and numerous rare tauopathies. A small molecule approach targeting tau aggregation was used to select and optimize compounds inhibiting tau self-association in vitro that have translated in vivo in preventive studies in htau and P301L tau JNPL3 mouse models of tauopathy. In this therapeutic treatment study, aged JNPL3 mice with pre-existing tau aggregates were used to evaluate the therapeutic effect of OLX-07010. The study had a Baseline group of mice aged 7 months, a vehicle, and two dose groups treated until 12 months by administration in feed. The primary endpoint of the study was the reduction of insoluble tau aggregates with statistical significance. The secondary endpoints were dose-dependent reduction of insoluble tau aggregates, reduction of soluble tau, and improvement of motor behavior. ELISAs and immunoblots were used to determine the levels of tau and its aggregated forms including self-associated tau and Sarkosyl insoluble tau. Effect on motor behavior, as measured by Rotarod assay, was also assessed between the treatment groups. At the end of treatment, reduced levels of self-associated tau, Sarkosyl insoluble tau aggregates, and overall levels of tau in the heat-stable fraction with statistical significance in the cortex were observed. Treatment prevented the accumulation of tau aggregates above baseline, and in parallel, treatment groups had improved motor behavior in a Rotarod assay compared to baseline and vehicle control groups, suggesting that treatment was rescuing motor impairment in aged mice. The functional and biochemical readouts suggest that this small molecule has potential for treating neurodegenerative diseases characterized by tau aggregation such as AD and progressive supranuclear palsy.
© 2025 Oligomerix, Inc. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
E.D., P.L., D.P., and J.M. are full‐time employees of Oligomerix Inc. and own stocks in the company. D.M. consults with In Med, MindImmune, SynapsDx, and Hesperos on projects unrelated to this work. The remaining authors declare no conflicts of interest.
Figures




References
-
- Buccarello, L. , Grignaschi G., Castaldo A. M., et al. 2017. “Sex Impact on Tau‐Aggregation and Postsynaptic Protein Levels in the P301L Mouse Model of Tauopathy.” Journal of Alzheimer's Disease 56: 1279–1292. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

