Costs of biomarker testing in advanced non-small cell lung cancer: a global study comparing next-generation sequencing and single-gene testing
- PMID: 40052485
- PMCID: PMC11886603
- DOI: 10.1002/2056-4538.70018
Costs of biomarker testing in advanced non-small cell lung cancer: a global study comparing next-generation sequencing and single-gene testing
Abstract
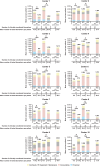
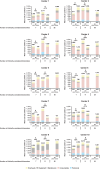
Current European/US guidelines recommend that molecular testing in advanced non-small cell lung cancer (aNSCLC) be performed using next-generation sequencing (NGS). However, the global uptake of NGS is limited, largely owing to reimbursement constraints. We compared real-world costs of NGS and single-gene testing (SGT) in nonsquamous aNSCLC. This observational study was conducted across 10 pathology centers in 10 different countries worldwide. Biomarker data collected via structured questionnaires (1 January-31 December 2021) were used to feed micro-costing analyses for three scenarios ['Starting Point' (SP; 2021-2022), 'Current Practice' (CP; 2023-2024), and 'Future Horizons' (FH; 2025-2028)] in both a real-world model, comprising all biomarkers tested by each center, and a standardized model, comprising the same sets of biomarkers across centers. Testing costs (including retesting) encompassed personnel costs, consumables, equipment, and overheads. Overall, 4,491 patients with aNSCLC were evaluated. Mean per-patient costs decreased for NGS relative to SGT over time, with real-world model costs 18% lower for NGS than for SGT in the SP scenario, and 26% lower for NGS than for SGT in the CP scenario. Mean per-biomarker costs also decreased over time for NGS relative to SGT. In the standardized model, the tipping point for the minimum number of biomarkers required for NGS to result in cost savings (per patient) was 10 and 12 in the SP and CP scenarios, respectively. Retesting had a negligible impact on cost analyses, and results were robust to variation in cost parameters. This study provides robust real-world global evidence for cost savings with NGS-based panels over SGT to evaluate predictive biomarkers in nonsquamous aNSCLC when the number of biomarkers to be tested exceeds 10. Widespread adoption of NGS may enable more efficient use of limited healthcare resources.
Keywords: NSCLC; cost comparison; next‐generation sequencing; precision medicine; predictive biomarker; single‐gene testing.
© 2025 The Author(s). The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures

References
-
- American Cancer Society . Cancer Facts & Figures 2024. [Accessed 13 January 2025]. Available from: https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐...
-
- Sholl LM, Cooper WA, Kerr KM, et al. In: IASLC Atlas of Molecular Testing for Targeted Therapy in Lung Cancer, Sholl LM, Cooper WA, Kerr KM, et al. (Eds). International Association for the Study of Lung Cancer, 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

