UNC-6/Netrin promotes both adhesion and directed growth within a single axon
- PMID: 40052533
- PMCID: PMC11888602
- DOI: 10.7554/eLife.100424
UNC-6/Netrin promotes both adhesion and directed growth within a single axon
Abstract
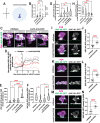
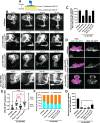
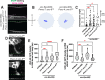
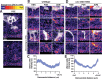
During development axons undergo long-distance migrations as instructed by guidance molecules and their receptors, such as UNC-6/Netrin and UNC-40/DCC. Guidance cues act through long-range diffusive gradients (chemotaxis) or local adhesion (haptotaxis). However, how these discrete modes of action guide axons in vivo is poorly understood. Using time-lapse imaging of axon guidance in C. elegans, we demonstrate that UNC-6 and UNC-40 are required for local adhesion to an intermediate target and subsequent directional growth. Exogenous membrane-tethered UNC-6 is sufficient to mediate adhesion but not directional growth, demonstrating the separability of haptotaxis and chemotaxis. This conclusion is further supported by the endogenous UNC-6 distribution along the axon's route. The intermediate and final targets are enriched in UNC-6 and separated by a ventrodorsal UNC-6 gradient. Continuous growth through the gradient requires UNC-40, which recruits UNC-6 to the growth cone tip. Overall, these data suggest that UNC-6 stimulates stepwise haptotaxis and chemotaxis in vivo.
Keywords: C. elegans; UNC-40/DCC; UNC-6/Netrin; axon guidance; chemotaxis; developmental biology; haptotaxis; neuroscience.
© 2024, Nichols et al.
Conflict of interest statement
EN, JL, KS No competing interests declared
Figures


Update of
- doi: 10.1101/2024.06.05.597665
- doi: 10.7554/eLife.100424.1
- doi: 10.7554/eLife.100424.2
Comment in
- doi: 10.7554/elife.106190
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

