A pilot study of a targeted cognitive intervention for cancer survivors
- PMID: 40053154
- PMCID: PMC11889051
- DOI: 10.1007/s00520-025-09321-z
A pilot study of a targeted cognitive intervention for cancer survivors
Abstract
Purpose: The primary aims of this four week pilot randomized clinical trial (RCT) involving a targeted cognitive intervention (TCI, n = 25) compared to an expectancy matched active control intervention (EMACI, n = 24), in a sample of cancer survivors were to: determine recruitment and retention rates; evaluate preliminary efficacy to improve three objective measures of cognitive function (i.e., attention, working memory, multi-tasking); evaluate adherence rates for and satisfaction with the interventions, and evaluate for treatment-related adverse events (e.g., nausea, motion sickness).
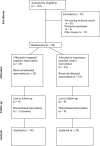
Methods: Cancer survivors were recruited from previous studies through email. Following a screening call, survivors who consented to participate were oriented to the study measures and procedures via Zoom. Survivors were randomized to the TCI or EMACI and mailed an iPad with the software for their specific intervention and the Adaptive Cognitive Evaluation Explorer (ACE-X, the objective measure of cognitive function). Survivors used the intervention for 25 min per day at least 5 days per week. Differences in objective measures of attention, working memory, and multi-tasking were evaluated using multilevel regression analyses.
Results: For the sustained attention measure, a significant cross-level interaction was found in favor of the TCI group. While improvements in multi-tasking occurred in both groups, while not statistically significant, the trend was larger for the TCI group. Equally important, in both groups, adherence with the intervention was high and adverse effects were minimal.
Conclusions: These preliminary findings provide promising evidence of feasibility, acceptability, and efficacy that warrant evaluation in a RCT with a larger sample of cancer survivors.
Keywords: Attention; Cancer; Cognitive impairment; Cognitive intervention; Multi-tasking; Working memory.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Committee on Human Research at the University of California, San Francisco. Consent to participate: Digital informed consent was obtained from the patients. Consent to publish: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Horowitz TS, Suls J, Trevino M (2018) A call for a neuroscience approach to cancer-related cognitive impairment. Trends Neurosci 41(8):493–496. 10.1016/j.tins.2018.05.001 - PubMed
-
- Rick O, Gerhardt A, Schilling G (2024) Cancer-related cognitive dysfunction: a narrative review for clinical practice. Oncol Res Treat 47(5):218–223. 10.1159/000538277 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

