Triclosan-Containing Sutures for the Prevention of Surgical Site Infection: A Systematic Review and Meta-Analysis
- PMID: 40053348
- PMCID: PMC11889475
- DOI: 10.1001/jamanetworkopen.2025.0306
Triclosan-Containing Sutures for the Prevention of Surgical Site Infection: A Systematic Review and Meta-Analysis
Abstract
Importance: International guidelines recommend the use of triclosan-containing sutures for the prevention of surgical site infections. However, controversy still remains about triclosan-containing suture use in clinical practice since several new randomized clinical trials (RCTs) have shown contradicting results.
Objective: To update a previous systematic review and meta-analysis of the association of triclosan-containing sutures with surgical site infections and explore the potential added value of new RCTs.
Data sources: PubMed, Embase, and Cochrane CENTRAL databases were searched from January 1, 2015, to March 14, 2023. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was followed.
Study selection: Published RCTs comparing triclosan-containing sutures with similar sutures without triclosan for the prevention of surgical site infections in any type of surgery were included.
Data extraction and synthesis: Two authors (H.J. and A.S.T.) independently extracted and pooled data in a random-effects (Mantel-Haenszel) model. The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation approach, and trial sequential analysis was used to estimate whether further studies would reveal different outcomes.
Main outcomes and measures: The primary outcome was the incidence of surgical site infections, expressed as relative risk (RRs) and corresponding 95% CIs. Secondary outcomes were the incidence of surgical site infections according to depth (superficial incisional, deep incisional, and organ/space) and adverse events related to triclosan-containing sutures.
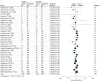
Results: The systematic review yielded 15 additional RCTs compared with a previous published review in 2017. A meta-analysis of 31 studies including 17 968 participants (62% male) undergoing various types of surgery was performed. Use of triclosan-containing sutures was associated with fewer surgical site infections compared with sutures without triclosan (RR, 0.75; 95% CI, 0.65-0.86). The certainty of evidence was moderate after downgrading for heterogeneity (τ2 = 0.04; I2 = 43%). In the trial sequential analysis of all trials and a sensitivity analysis excluding studies with a high risk of bias, the cumulative z curve crossed the trial sequential monitoring boundary for benefit, confirming the robustness of the summary effect estimate.
Conclusions and relevance: This updated meta-analysis found moderate-certainty evidence that wound closure with triclosan-containing sutures was associated with a lower risk of surgical site infections. The trial sequential analysis suggests that future trials that would change these findings are improbable.
Conflict of interest statement
Figures


References
-
- National Healthcare Safety Network . Surgical Site Infection Event (SSI). Centers for Disease Control and Prevention. Published January 2023. Accessed August 15, 2024. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

