COVID-19 Outcomes Among Adults Receiving Electronic Nudges to Increase Influenza Vaccination: A Prespecified Secondary Analysis of the NUDGE-FLU Trial
- PMID: 40053354
- PMCID: PMC11889467
- DOI: 10.1001/jamanetworkopen.2025.0320
COVID-19 Outcomes Among Adults Receiving Electronic Nudges to Increase Influenza Vaccination: A Prespecified Secondary Analysis of the NUDGE-FLU Trial
Plain language summary
This secondary analysis of a randomized clinical trial investigates whether electronic nudging letters to increase influenza vaccination have an effect on COVID-19 vaccination, infection, and hospitalization.
Conflict of interest statement
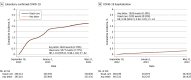
Figures

References
-
- World Health Organization . Global influenza strategy 2019-2030. World Health Organization. 2019. Accessed December 20, 2022. https://apps.who.int/iris/handle/10665/311184
-
- CDC . Flu vaccination coverage, United States, 2022–23 influenza season | FluVaxView | seasonal influenza (flu). Published October 10, 2023. Accessed November 27, 2023. https://www.cdc.gov/fluvaxview/coverage-by-season/2022-2023.html
-
- Altmann S, Grunewald A, Radbruch J. Interventions and cognitive spillovers. Rev Econ Stud. 2022;89(5):2293-2328. doi: 10.1093/restud/rdab087 - DOI
LinkOut - more resources
Full Text Sources

