Outcomes in people with HIV who resume or switch to bictegravir/emtricitabine/tenofovir alafenamide following a treatment interruption
- PMID: 40053475
- PMCID: PMC12237111
- DOI: 10.1097/QAD.0000000000004171
Outcomes in people with HIV who resume or switch to bictegravir/emtricitabine/tenofovir alafenamide following a treatment interruption
Abstract
Objective: Treatment adherence remains critical in maintaining HIV RNA suppression on antiretroviral therapy. High genetic barrier regimens constructed with three long half-life agents may prevent resistance emergence and can be potentially started or restarted after antiretroviral treatment interruption.
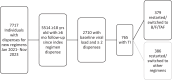
Methods: Data from the TRIO US HIV cohort were used to identify adult people with HIV initiating a new ART regimen from January 2021 to November 2023 and describe prevalence of treatment interruptions (defined as ≥90 days without dispensed ART). Virologic outcomes were assessed among those restarting or switching to B/F/TAF after treatment interruption.
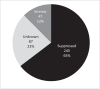
Results: Of 2710 people with HIV, 765 (28%) experienced treatment interruption. Compared to individuals without treatment interruptions, those with treatment interruptions had higher proportion of women (24 vs. 19%), Black race (50 vs. 35%), substance use (14 vs. 9%), CD4 + cell count less than 200 cells/mm 3 (15 vs. 8%) and lower proportion with commercial insurance (48 vs. 62%) or virologic suppression at initiation (76 vs. 85%). Among 379 who restarted or switched to B/F/TAF following treatment interruption, 245 (65%) were suppressed at restart; 137 (56%) had at least one viral load after treatment interruption, of whom 129 (94%) maintained suppression. Of 87 with unknown viral status at restart, 46 (53%) had at least one viral load during follow-up, of whom 44 (96%) achieved suppression. Among 47 viremic at restart, 27 (57%) had at least one viral load after treatment interruption. Of them, 70% were suppressed during follow-up. No integrase inhibitor resistance emergence was observed.
Conclusion: High levels of suppression following treatment interruption may suggest B/F/TAF regimen forgiveness making it an appropriate choice for treatment switch or restart.
Keywords: adherence; antiretroviral resistance; antiretroviral therapy; bictegravir; therapy restart; treatment interruptions; viral suppression.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- What's new: adult and adolescent ARV HIV Clinical Guidelines | NIH. September 12, 2024. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult.... [Accessed 5 December 2024].
-
- Shuter J. Forgiveness of nonadherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother 2008; 61:769–773. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials