Rationale and design of the Renal Lifecycle trial assessing the effect of dapagliflozin on cardiorenal outcomes in severe chronic kidney disease
- PMID: 40053493
- PMCID: PMC12394133
- DOI: 10.1093/ndt/gfaf046
Rationale and design of the Renal Lifecycle trial assessing the effect of dapagliflozin on cardiorenal outcomes in severe chronic kidney disease
Abstract
Background: Several clinical trials have shown beneficial effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on kidney disease progression and cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD) with and without type 2 diabetes mellitus. However, some subgroups of patients with CKD have been excluded from participation in these trials, such as patients with severely impaired kidney function, patients on dialysis and kidney transplant recipients.
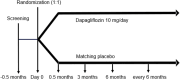
Methods: The Renal Lifecycle trial (NCT05374291) is a pragmatic, international, multicentre, investigator-initiated, randomized, placebo-controlled clinical trial planned to enrol ≈1500 patients with an estimated glomerular filtration rate (eGFR) ≤25 ml/min/1.73 m2, on haemodialysis or peritoneal dialysis or after a kidney transplant and an eGFR ≤45 ml/min/1.73 m2, who will be randomized 1:1 to receive either dapagliflozin 10 mg once daily or matching placebo.
Results: The primary endpoint is a composite of heart failure hospitalization, all-cause mortality or, for those not on dialysis, kidney failure (start of dialysis >1 month, receiving a kidney transplant or death due to kidney failure). The trial is event driven, indicating that it will end after 468 first primary endpoint events have occurred, with a power of 80% and an α of 0.05 to detect a 25% relative risk reduction assuming an annual 12.5% incidence of the primary outcome. The secondary endpoints include a separate analysis of the incidence of each component of the primary endpoint in the overall trial population as well as the incidence of the combined primary endpoint in each of the three subgroups of patients. Other (exploratory) endpoints are efficacy, safety, tolerability, health-related quality of life and cognition.
Conclusion: The Renal Lifecycle trial aims to investigate the effects of the SGLT2 inhibitor dapagliflozin compared with placebo on the incidence of kidney failure, heart failure, mortality and safety in three subgroups of patients with advanced CKD.
Keywords: SGLT2 inhibitor; chronic kidney disease; dialysis; kidney failure; kidney transplantation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
P.v.d.M. has received research grants and/or consulting fees from Novartis, Vifor Pharma, Pharmacosmos, Ionis, AstraZeneca, Pharma Nord, Novo Nordisk Boehringer Ingelheim, Bayer and Pfizer, all paid to his institution. M.H. has received research grants from Bayer and AstraZeneca. D.K. has received consulting fees from AstraZeneca, Bayer, Sangamo-Tx, ArgenX, Takeda, GSK and Astellas. SVB declared to have received consulting fees/honoraria from Bayer, AstraZeneca, Pfizer, GSK, Novo Nordisk and Vifor Pharma. C.A. has received honoraria from Novo Nordisk, Amgen and AstraZeneca. M.G.V. declared to have received research support/and or consulting fees from Fresenius Medical care, Admesy and CSL-Vifor, Dutch Kidney Foundation and Health Holland, Astra-Zeneca and Boehringer-Ingolheim. A.C.A. has received speaking fees from Baxter, Fresenius Medical Care and Cablon Medical and grant support from the Dutch Kidney Foundation and Baxter outside the submitted work. J.v.d.N. has received consulting fees from AstraZeneca, Novo Nordisk and Vifor Pharma. C.W. has received honoraria for steering committee and advisory board participation as well as lecturing from AstraZeneca, Boehringer Ingelheim, Bayer, Lilly, MSD and Sanofi. J.L.G. has received funding to conduct clinical trials from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk (all to the INCLIVA Research Institute); consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk; and payment of honoraria for lectures from AstraZeneca, Novo Nordisk, Bayer, Menarini, Boehringer Ingelheim and Lilly. A.Y.W. has received honoraria from AstraZeneca, Bayer AG and Fresenius Kabi and served as an advisory board member for Fresenius Kabi. A.P.J.V. declared to have received consulting fees/honoraria of CSL Behring, Hansa, Neovvi, Takeda, Sanofi, Astellas, Chiesi and Sandoz. All was paid to the institution. H.J.L.H declared to have Advisory Board contracts with AstraZeneca, Alexion, Alnylum, Biocity, Bayer, Boehringer Ingelheim, CSL-Behring, Dimerix, Eli-Lilly, Idorsia, NovoNordisk, Novartis, Roche, Travere Therapeutics and received payments for lectures from AstraZeneca, Bayer, NovoNordisk. L.J. declared to have received research grant of the Dutch Kidney foundation and Speaker fee for lectures PD Essenties and PD University by Vantive. M.W. declared to have received research grants and/or consulting fees of the Canadian Institutes of Health Research, British Heart Foundation, Medical Research Future Fund, Health Research Council, Hamilton Academic Health Sciences Organization, Otsuka Pharmaceuticals, GSK, Bayer, Vistera and Alexion. S.P.B. declared to have received payment/honoraria of Astellas, Chiesi. Wages of the study physician W.M.B. were paid by AstraZeneca. All payments were made to the institution. R.T.G. declared to have received grants of Astra-Zeneca, Abbvie, Bayer, Calico, GSK, Otsuka, Roche, Siemens, Travere, Vertex. The other members of the committees and authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous