Citrulline regulates macrophage metabolism and inflammation to counter aging in mice
- PMID: 40053596
- PMCID: PMC11887811
- DOI: 10.1126/sciadv.ads4957
Citrulline regulates macrophage metabolism and inflammation to counter aging in mice
Abstract
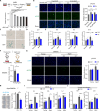
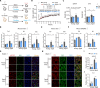
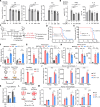
Metabolic dysregulation and altered metabolite concentrations are widely recognized as key characteristics of aging. Comprehensive exploration of endogenous metabolites that drive aging remains insufficient. Here, we conducted an untargeted metabolomics analysis of aging mice, revealing citrulline as a consistently down-regulated metabolite associated with aging. Systematic investigations demonstrated that citrulline exhibited antiaging effects by reducing cellular senescence, protecting against DNA damage, preventing cell cycle arrest, modulating macrophage metabolism, and mitigating inflammaging. Long-term citrulline supplementation in aged mice yielded beneficial effects and ameliorated age-associated phenotypes. We further elucidated that citrulline acts as an endogenous metabolite antagonist to inflammation, suppressing proinflammatory responses in macrophages. Mechanistically, citrulline served as a potential inhibitor of mammalian target of rapamycin (mTOR) activation in macrophage and regulated the mTOR-hypoxia-inducible factor 1α-glycolysis signaling pathway to counter inflammation and aging. These findings underscore the significance of citrulline deficiency as a driver of aging, highlighting citrulline supplementation as a promising therapeutic intervention to counteract aging-related changes.
Figures



References
-
- Lopez-Otin C., Galluzzi L., Freije J. M. P., Madeo F., Kroemer G., Metabolic control of longevity. Cell 166, 802–821 (2016). - PubMed
-
- Lopez-Otin C., Blasco M. A., Partridge L., Serrano M., Kroemer G., Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023). - PubMed
-
- Savini M., Folick A., Lee Y. T., Jin F., Cuevas A., Tillman M. C., Duffy J. D., Zhao Q., Neve I. A., Hu P. W., Yu Y., Zhang Q., Ye Y., Mair W. B., Wang J., Han L., Ortlund E. A., Wang M. C., Lysosome lipid signalling from the periphery to neurons regulates longevity. Nat. Cell Biol. 24, 906–916 (2022). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

