Pembrolizumab in Combination with Binimetinib in Patients with Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer
- PMID: 40053697
- PMCID: PMC12079099
- DOI: 10.1158/1078-0432.CCR-24-3001
Pembrolizumab in Combination with Binimetinib in Patients with Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer
Abstract
Purpose: Activation of the RAS/MAPK pathway is associated with reduced tumor-infiltrating lymphocytes and poor outcomes in triple-negative breast cancer. Previous studies demonstrated that inhibition of the MAPK pathway with a MEK inhibitor is synergistic with immune checkpoint inhibitors.
Patients and methods: We conducted a phase I/II trial of pembrolizumab and binimetinib in patients with metastatic triple-negative breast cancer with ≤3 prior lines of therapy. There were two dose levels (DL) with binimetinib at 45 mg at DL 0 and 30 mg at DL -1.
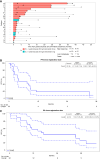
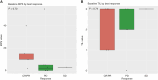
Results: The recommended phase II dose was the standard dose of pembrolizumab with binimetinib 30 mg twice daily. The objective response rate (ORR) was 30.4%, with a numerically higher ORR in patients without liver metastasis at 45.5%. Among patients who achieved objective responses, 80% had a duration of response >12 months and ongoing even after stopping treatment (5.4-69.0 months). Patients with PD-L1-positive tumors (modified proportion score ≥10) were more likely to respond with an ORR of 66.7%. However, clinical benefit was observed in 25% of patients with PD-L1-negative tumors. Consistent with preclinical studies, four of six patients with clinical benefit had either increased PD-L1 or decreased p-ERK expressions in serial circulating cancer-associated macrophage-like cells after starting binimetinib.
Conclusions: Pembrolizumab and binimetinib at 30 mg are safe with manageable toxicities. Promising activity was observed in patients without liver metastases. Future larger clinical trials are warranted to further evaluate the efficacy of this chemotherapy-free combination.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Chumsri reports grants from Merck & Co. and Pfizer during the conduct of the study, as well as personal fees from Novartis, AstraZeneca, Daiichi Sankyo, Genentech, Puma Biotechnology, Eisai, and Seagen outside the submitted work. D. Adams reports other support from Creatv MicroTech during the conduct of the study, as well as other support from Creatv MicroTech outside the submitted work and a patent for Provisional Filed pending to Creatv MicroTech. P. Advani reports other support from AstraZeneca, DSI, Pfizer/Seagen, Atossa Therapeutics, Modulation Therapeutics, Biovica, Loxo Lilly, Sermonix, Gilead, Menarini Stemline, Puma, and Elephas and personal fees from Biovica, DSI, AstraZeneca, Hesian labs, Elephas, GE HealthCare, Guardant, Menarini, Breathe BioMedical, and MJH Life Sciences outside the submitted work. K.L. Knutson reports nonfinancial support from Merck outside the submitted work, as well as a patent for PCT/US24/12603 pending. No disclosures were reported by the other authors.
Figures
References
-
- Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, et al. . Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 2022;387:217–26. - PubMed
-
- Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. . Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397–404. - PubMed
-
- Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. . Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:405–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

