Robust generation of photoreceptor-dominant retinal organoids from porcine induced pluripotent stem cells
- PMID: 40054472
- PMCID: PMC12069893
- DOI: 10.1016/j.stemcr.2025.102425
Robust generation of photoreceptor-dominant retinal organoids from porcine induced pluripotent stem cells
Abstract
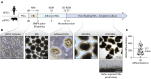
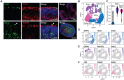
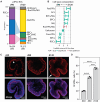
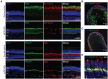
Outer retinal degenerative diseases (RDDs) and injuries leading to photoreceptor (PR) loss are prevailing causes of blindness worldwide. While significant progress has been made in the manufacture of human pluripotent stem cell (hPSC)-derived PRs, robust production of pluripotent stem cell (PSC)-PRs from swine, a popular preclinical large animal model, would provide an avenue to collect conspecific functional and safety data to complement human xenograft studies. Toward this goal, we describe the highly efficient generation of PR-dominant porcine induced PSC (piPSC)-derived retinal organoids (ROs) using modifications of our established hPSC-RO differentiation protocol. Porcine iPSC-ROs were characterized using immunocytochemistry (ICC) and single-cell RNA sequencing (scRNA-seq), which revealed the presence and maturation of major neural retina cell types, including PRs and retinal ganglion cells, which possess molecular signatures akin to those found in hPSC-ROs. In late piPSC-ROs, a highly organized outer neuroepithelium was observed with rods and cones possessing outer segments and axon terminals expressing pre-synaptic markers adjacent to dendritic terminals of bipolar cells. The existence of piPSC lines and protocols that support reproducible, scalable production of female and male ROs will facilitate transplant studies in porcine models of retinal injury and RDDs unconfounded by immunological and evolutionary incompatibilities inherent to human xenografts.
Keywords: allograft; cell transplantation; iPSC-derived retinal cells; neural retina; photoreceptors; porcine iPSCs; retinal differentiation; retinal organoids; scRNA-seq.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Barone F., Muscatello L.V., Ventrella D., Elmi A., Romagnoli N., Mandrioli L., Maya-Vetencourt J.F., Bombardi C., Mete M., Sarli G., et al. The porcine iodoacetic acid model of retinal degeneration: Morpho-functional characterization of the visual system. Exp. Eye Res. 2020;193 doi: 10.1016/j.exer.2020.107979. - DOI - PubMed
-
- Barone F., Amaral J., Bunea I., Farnoodian M., Gupta R., Gupta R., Baker D., Phillips M.J., Blanch R.J., Maminishkis A., et al. American Society for Clinical Investigation; 2023. A Versatile Laser-Induced Porcine Model of Outer Retinal and Choroidal Degeneration for Preclinical Testing. - PMC - PubMed
-
- Capowski E.E., Samimi K., Mayerl S.J., Phillips M.J., Pinilla I., Howden S.E., Saha J., Jansen A.D., Edwards K.L., Jager L.D., et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Dev. Camb. Engl. 2019;146 doi: 10.1242/dev.171686. - DOI - PMC - PubMed
-
- Chandler M.J., Smith P.J., Samuelson D.A., MacKay E.O. Photoreceptor density of the domestic pig retina. Vet. Ophthalmol. 1999;2:179–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

