Proposal of discontinuation criteria of atezolizumab plus bevacizumab after curative conversion therapy for unresectable early-to-intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study
- PMID: 40055288
- PMCID: PMC12095402
- DOI: 10.1007/s00535-025-02233-z
Proposal of discontinuation criteria of atezolizumab plus bevacizumab after curative conversion therapy for unresectable early-to-intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study
Abstract
Background: Achieving complete response (CR) is a desirable goal in early-to-intermediate-stage hepatocellular carcinoma (HCC). While systemic and locoregional therapies show promise, optimal drug discontinuation criteria remain unclear. This study aims to investigate drug-off criteria for atezolizumab plus bevacizumab as a proof-of-concept study.
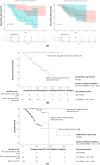
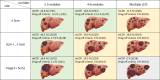
Methods: This retrospective multicenter study included child-pugh class A patients with unresectable HCC without extrahepatic spread or macrovascular invasion who received atezolizumab plus bevacizumab as first-line therapy. Modified clinical CR (mCCR) was defined as CR per mRECIST with sustained normal alpha-fetoprotein (AFP) levels (< 10.0 ng/dl). Recurrence-free survival (RFS) and overall survival (OS) were analyzed based on the "drug-off" criteria defined by following: (1) mRECIST CR with locoregional therapies, (2) sustained normalization of AFP/AFP-L3/ des-gamma-carboxy prothrombin (DCP) for 12-24 weeks, and (3) complete tumor vascularity disappearance by contrast-enhanced ultrasonography (CEUS) or pathological curative resection.
Results: The median follow-up was 16.5 months (95% CI 15.2-17.8). Among 51 patients achieving mCCR, 11 underwent surgery, with pathological CR in three cases. In contrast, viable lesions were observed in 7 of 40 cases assessed using CEUS. All patients meeting the drug-off criteria (n = 9) showed no recurrence and none of them experienced mortality, while 45.2% (19/42) of those not meeting the criteria experienced recurrence (median RFS: 12.8 months, p = 0.007). The median OS was not reached in dug-off criteria met patients (n = 9), 37.7 months (95% CI: NA) in non-criteria met patients (n = 42), and 27.1 months (95% CI 16.7-37.6) in non-mCCR patients (n = 184) (p < 0.001).
Conclusion: In patients with unresectable and TACE-unsuitable early-to-intermediate-stage HCC who met the drug-off criteria, significantly improved RFS and OS were observed compared those who did not meet the criteria. However, further validation studies are required to confirm the utility of the criteria.
Keywords: Carcinoma, Hepatocellular [MH]; Conversion therapy; Drug-off; Immune checkpoint inhibitors [MH]; Treatment outcome [MH].
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: T.A. received lecture fees; Eisai. M.K. received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, GE Healthcare, AbbVie, Astellas Pharma, Eisai, Eli Lilly, and AstraZeneca. He has also received grants and personal fees from Eisai, Roche, Chugai, and AstraZeneca. N.N. No relevant conflicts of interest to disclose. K.U. received honoraria from Eisai, Takeda and Chugai. K.T. received lecture fees; Chugai, Eisai, AstraZeneca, Takeda. T.T.: received lecture fees; Abbvie, Chugai and Eisai. M.M. No relevant conflicts of interest to disclose. H.C. No relevant conflicts of interest to disclose. M.T. No relevant conflicts of interest to disclose. S.H. No relevant conflicts of interest to disclose. H.I. No relevant conflicts of interest to disclose. Y.M. No relevant conflicts of interest to disclose. H.K. No relevant conflicts of interest to disclose. N.N. No relevant conflicts of interest to disclose. A.H.: received lecture fees; Chugai, AstraZeneca and Eli Lilly. T.T. No relevant conflicts of interest to disclose. J.T. received lecture fees; AstraZeneca. A.N. No relevant conflicts of interest to disclose. S.K.: received lecture fees; Abbvie. C.O. received lecture fees; Chugai. T.H. received lecture fees; Eisai. T.I. No relevant conflicts of interest to disclose. K.K. No relevant conflicts of interest to disclose. A.T. No relevant conflicts of interest to disclose. I.M. No relevant conflicts of interest to disclose. M.H. No relevant conflicts of interest to disclose. M.K. received lecture fees; Eisai, Bayer, Lilly, Chugai, Takeda, AstraZeneca. T.K.: received lecture fees; Eisai. N.I. received lecture fees; Chugai, AstraZeneca, Eisai, Takeda. Ethical approval: This study was approved by the Medical Ethics Committee of Kindai University Hospital (Approval R02-258) in 2020, and written informed consent was obtained from all patients.
Figures



References
-
- Hasegawa K, Takemura N, Yamashita T, Watadani T, Kaibori M, Kubo S, Shimada M, Nagano H, Hatano E, Aikata H, Iijima H, Ueshima K, Ohkawa K, Genda T, Tsuchiya K, Torimura T, Ikeda M, Furuse J, Akahane M, Kobayashi S, Sakurai H, Takeda A, Murakami T, Motosugi U, Matsuyama Y, Kudo M, Tateishi R. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol Res. 2023;53(5):383–90. - PubMed
-
- Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, Tateishi R, Nakashima O, Murakami T, Matsuyama Y, Takahashi A, Miyata H, Kubo S. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012–2013). Hepatol Res. 2022;52(1):5–66. - PubMed
-
- Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26(2):142–7. - PubMed
-
- Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Kubo S, Sakamoto M, Nakashima O, Kumada T, Kokudo N, Liver Cancer Study Group of Japan. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017;66(2):510–7. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

