An atypical atherogenic chemokine that promotes advanced atherosclerosis and hepatic lipogenesis
- PMID: 40055309
- PMCID: PMC11889166
- DOI: 10.1038/s41467-025-57540-z
An atypical atherogenic chemokine that promotes advanced atherosclerosis and hepatic lipogenesis
Abstract
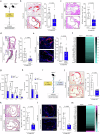
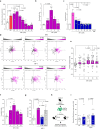
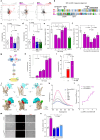
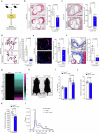
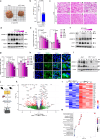
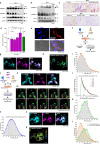
Atherosclerosis is the underlying cause of myocardial infarction and ischemic stroke. It is a lipid-triggered and cytokine/chemokine-driven arterial inflammatory condition. We identify D-dopachrome tautomerase/macrophage migration-inhibitory factor-2 (MIF-2), a paralog of the cytokine MIF, as an atypical chemokine promoting both atherosclerosis and hepatic lipid accumulation. In hyperlipidemic Apoe-/- mice, Mif-2-deficiency and pharmacological MIF-2-blockade protect against lesion formation and vascular inflammation in early and advanced atherogenesis. MIF-2 promotes leukocyte migration, endothelial arrest, and foam-cell formation, and we identify CXCR4 as a receptor for MIF-2. Mif-2-deficiency in Apoe-/- mice leads to decreased plasma lipid levels and suppressed hepatic lipid accumulation, characterized by reductions in lipogenesis-related pathways, tri-/diacylglycerides, and cholesterol-esters, as revealed by hepatic transcriptomics/lipidomics. Hepatocyte cultures and FLIM-FRET-microscopy suggest that MIF-2 activates SREBP-driven lipogenic genes, mechanistically involving MIF-2-inducible CD74/CXCR4 complexes and PI3K/AKT but not AMPK signaling. MIF-2 is upregulated in unstable carotid plaques from atherosclerotic patients and its plasma concentration correlates with disease severity in patients with coronary artery disease. These findings establish MIF-2 as an atypical chemokine linking vascular inflammation to metabolic dysfunction in atherosclerosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.B., R.B., and C.W. are co-inventors of patents covering anti-MIF strategies for inflammatory and cardiovascular diseases. C.Ko., A.K., O.E., and J.B. are co-inventors of a patent application covering MIF-binding CXCR4 ectodomain mimics for inflammatory and cardiovascular diseases. The remaining authors declare no competing interests.
Figures


References
-
- Joseph, P. et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ. Res.121, 677–694 (2017). - PubMed
-
- Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature473, 317–325 (2011). - PubMed
-
- Virani, S. S. et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation141, e139–e596 (2020). - PubMed
-
- Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med.340, 115–126 (1999). - PubMed
-
- Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med.17, 1410–1422 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

