Antibiofilm activity of Plumbagin against Staphylococcus aureus
- PMID: 40055436
- PMCID: PMC11889106
- DOI: 10.1038/s41598-025-92435-5
Antibiofilm activity of Plumbagin against Staphylococcus aureus
Abstract
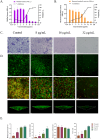
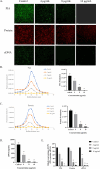
In chronic infections caused by Staphylococcus aureus, biofilm is a major virulence factor. In Staphylococcus aureus biofilms, bacteria are embedded in a matrix of extracellular polymeric substances and are highly tolerant to antimicrobial drugs. However, the lack of effective solutions to inhibit biofilm formation remains a challenge, and the mechanism of inhibition of biofilm formation targeting extracellular polymeric substances is unclear. The aim of the present study was to investigate the inhibitory mechanisms of Plumbagin against Staphylococcus aureus biofilms formation by affecting secretion of extracellular polymeric substances using the high-content screening. Our results showed Plumbagin (16 µg/mL) inhibited biofilm formation, revealing a significant reduction in both biomass and bacterial metabolic activity, and disrupted the biofilm structure, leading to a significant decrease in both biological volume and average thickness (P ≤ 0.01). High-content screening imaging indicated that the Plumbagin treatment induced alterations in the extracellular polymeric substances of Staphylococcus aureus biofilm, significantly reducing the quantities of extracellular polysaccharide, proteins and extracellular DNA. Interestingly, extracellular DNA within the matrix was found to be the most sensitive to Plumbagin treatment. Extracellular DNA formation was significantly inhibited at a concentration of 4 µg/mL, whereas the inhibition of extracellular polysaccharide and proteins required a higher concentration of 8 µg/mL. Overall, these results demonstrated the inhibitory effects of Plumbagin on Staphylococcus aureus biofilm formation and extracellular polymeric substances secretion, suggesting that extracellular DNA may be a potential target for the anti-biofilm activity of Plumbagin. These findings will provide new insights into the mode of action of Plumbagin in treating infections caused by Staphylococcus aureus biofilms.
Keywords: Staphylococcus aureus; Biofilm; Extracellular polymeric substance; High-content screening; Plumbagin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
The antimicrobial and antibiofilm effects of gentamicin, imipenem, and fucoidan combinations against dual-species biofilms of Staphylococcus aureus and Acinetobacter baumannii isolated from diabetic foot ulcers.Ann Clin Microbiol Antimicrob. 2024 Nov 15;23(1):101. doi: 10.1186/s12941-024-00760-w. Ann Clin Microbiol Antimicrob. 2024. PMID: 39548455 Free PMC article.
-
Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans.Int J Med Microbiol. 2016 Jun;306(4):237-48. doi: 10.1016/j.ijmm.2016.05.004. Epub 2016 May 10. Int J Med Microbiol. 2016. PMID: 27212459
-
Enhanced penetration and biofilm eradication by sophorolipid micelles encapsulating Honokiol: a comprehensive solution for biofilm-associated lung infections.J Nanobiotechnology. 2025 Feb 3;23(1):76. doi: 10.1186/s12951-025-03144-0. J Nanobiotechnology. 2025. PMID: 39901249 Free PMC article.
-
Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies.Microbiol Mol Biol Rev. 2020 Aug 12;84(3):e00026-19. doi: 10.1128/MMBR.00026-19. Print 2020 Aug 19. Microbiol Mol Biol Rev. 2020. PMID: 32792334 Free PMC article. Review.
-
Vision for medicine: Staphylococcus aureus biofilm war and unlocking key's for anti-biofilm drug development.Microb Pathog. 2018 Oct;123:339-347. doi: 10.1016/j.micpath.2018.07.002. Epub 2018 Jul 3. Microb Pathog. 2018. PMID: 30057355 Review.
References
-
- James, G. A. et al. Biofilms in chronic wounds. Wound Repair. Regen.16, 37–44 (2008). - PubMed
-
- Foster, T. J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev.41, 430–449 (2017). - PubMed
-
- Götz, F. Staphylococcus and biofilms. Mol. Microbiol.43, 1367–1378 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- 22HHZYSS00003/the Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine
- ZYYCXTD-D-202002/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- 20501/KH2145/the Henan Liwei Biological Pharmaceutical Co., Ltd.
- 20501/KH2185/the Anhui Tiancien Biotechnology Co., Ltd.
LinkOut - more resources
Full Text Sources
Medical

