Extravascular coagulation regulates haemostasis independently of activated platelet surfaces in an in vivo mouse model
- PMID: 40055470
- PMCID: PMC11889134
- DOI: 10.1038/s42003-025-07838-x
Extravascular coagulation regulates haemostasis independently of activated platelet surfaces in an in vivo mouse model
Abstract
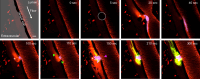
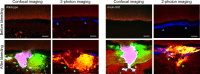
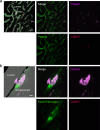
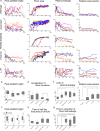
While the conventional understanding of haemostatic plug formation is that coagulation proceeds efficiently on the surface of activated platelets at the vascular injury site to form a robust haemostatic plug, this understanding does not explain the clinical reality that platelet dysfunction results in a mild bleeding phenotype, whereas coagulation disorders exhibit severe bleeding phenotypes, particularly in deep tissues. Here, we introduce an in vivo imaging method to observe internal bleeding and subsequent haemostatic plug formation in mice and report that haemostatic plug formation after internal bleeding, coagulation occurs primarily outside the blood vessel rather than on platelets. Experiments in mice with impaired platelet surface coagulation, depleted platelets, haemophilia A or reduced tissue factor expression suggest that this extravascular coagulation triggers and regulates haemostatic plug formation. Our discovery of the important role of extravascular coagulation in haemostasis may contribute to refining the treatment of haemostatic abnormalities and advancing antithrombotic therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: This study was funded by Chugai Pharmaceutical. The authors declare the following competing interests: A.S., K.T., Naoki M., S.H., R.K., Y.N., T.S., K.N., and M.S.: Members of the Medicinal Biology of Thrombosis and Haemostasis established by Nara Medical University and Chugai Pharmaceutical Co., Ltd. Naoki M., S.H., R.K., Y.N., and T.S.: Employees of Chugai Pharmaceutical Co., Ltd. Naoki M., S.H. and R.K.: Ownership of stock by Chugai Pharmaceutical Co., Ltd. M.S.: Patents for inventions related to products of Chugai Pharmaceutical Co., Ltd. K.T.: Grants or research support from the Japan Blood Products Organization, the Mother and Child Health Foundation and Novo Nordisk Pharma. M.S.: Takeda Pharmaceutical Co., Ltd., and CSL Behring; honoraria or consultation fees from Chugai Pharmaceutical Co., Ltd.; speaker bureau from Chugai Pharmaceutical Co., Ltd.; CSL Behring, Sanofi, Bayer, Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ltd., Pfizer, and Fujimoto Seiyaku Corp. K.N.: Representative of Medicinal Biology of Thrombosis and Haemostasis collaborative research laboratory; research support from Chugai Pharmaceutical Co., Ltd.; grants or research support from Chugai Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; KM Biologics Co., Ltd.; Sanofi Co., Ltd.; Novo Nordisk Pharma Co., Ltd.; Bayer Co., Ltd.; AbbVie GK LLC; Janssen Pharmaceutical K.K. Co., Ltd.; honouraria or consultation fees from Chugai Pharmaceutical Co., Ltd.; Sanofi Co., Ltd.; and CSL Behring.
Figures





References
-
- Hoffman, M. Remodeling the blood coagulation cascade. J. Thromb. Thrombolysis16, 17–20 (2003). - PubMed
-
- Dahlbäck, B. Blood coagulation. Lancet355, 1627–1632 (2000). - PubMed
-
- Kjalke, M. et al. Active site-inactivated factors VIIa, Xa, and IXa inhibit individual steps in a cell-based model of tissue factor-initiated coagulation. Thromb. Haemost.80, 578–584 (1998). - PubMed
-
- Oliver, J. A., Monroe, D. M., Roberts, H. R. & Hoffman, M. Thrombin activates factor XI on activated platelets in the absence of factor XII. Arterioscler. Thromb. Vasc. Biol.19, 170–177 (1999). - PubMed
-
- Bolton-Maggs, P. H. B. & Pasi, K. J. Haemophilias A and B. Lancet361, 1801–1809 (2003). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

