A torpor-like state in mice slows blood epigenetic aging and prolongs healthspan
- PMID: 40055478
- PMCID: PMC11922754
- DOI: 10.1038/s43587-025-00830-4
A torpor-like state in mice slows blood epigenetic aging and prolongs healthspan
Abstract
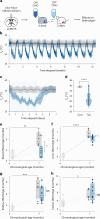
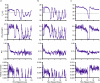
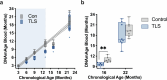
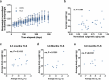
Torpor and hibernation are extreme physiological adaptations of homeotherms associated with pro-longevity effects. Yet the underlying mechanisms of how torpor affects aging, and whether hypothermic and hypometabolic states can be induced to slow aging and increase healthspan, remain unknown. Here we demonstrate that the activity of a spatially defined neuronal population in the preoptic area, which has previously been identified as a torpor-regulating brain region, is sufficient to induce a torpor-like state (TLS) in mice. Prolonged induction of TLS slows epigenetic aging across multiple tissues and improves healthspan. We isolate the effects of decreased metabolic rate, long-term caloric restriction, and decreased core body temperature (Tb) on blood epigenetic aging and find that the decelerating effect of TLSs on aging is mediated by decreased Tb. Taken together, our findings provide novel mechanistic insight into the decelerating effects of torpor and hibernation on aging and support the growing body of evidence that Tb is an important mediator of the aging processes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S. Horvath and R.T.B. are founders of the non-profit Epigenetic Clock Development Foundation, which licenses several patents from UC Regents including a patent on the mammalian methylation array platform. These patents list S. Horvath as inventor. V.G.S. serves as an advisor to and/or has equity in Branch Biosciences, Ensoma, and Cellarity, all unrelated to this work. S. Hrvatin is an advisor to Apertura LLC, unrelated to this work. The other authors declare no competing interests.
Figures








Update of
-
A torpor-like state (TLS) in mice slows blood epigenetic aging and prolongs healthspan.bioRxiv [Preprint]. 2024 Mar 25:2024.03.20.585828. doi: 10.1101/2024.03.20.585828. bioRxiv. 2024. Update in: Nat Aging. 2025 Mar;5(3):437-449. doi: 10.1038/s43587-025-00830-4. PMID: 38585858 Free PMC article. Updated. Preprint.
Similar articles
-
The cold truth: torpor as a confound in studies of caloric restriction.J Comp Physiol B. 2025 Jun;195(3):263-276. doi: 10.1007/s00360-025-01616-1. Epub 2025 Jun 9. J Comp Physiol B. 2025. PMID: 40488879 Free PMC article. Review.
-
A torpor-like state (TLS) in mice slows blood epigenetic aging and prolongs healthspan.bioRxiv [Preprint]. 2024 Mar 25:2024.03.20.585828. doi: 10.1101/2024.03.20.585828. bioRxiv. 2024. Update in: Nat Aging. 2025 Mar;5(3):437-449. doi: 10.1038/s43587-025-00830-4. PMID: 38585858 Free PMC article. Updated. Preprint.
-
Brainstem catecholaminergic neurons induce torpor during fasting by orchestrating cardiovascular and thermoregulation changes.Nat Commun. 2025 Jul 1;16(1):5954. doi: 10.1038/s41467-025-61179-1. Nat Commun. 2025. PMID: 40593766 Free PMC article.
-
Metabolic hormone FGF21 is induced in ground squirrels during hibernation but its overexpression is not sufficient to cause torpor.PLoS One. 2013;8(1):e53574. doi: 10.1371/journal.pone.0053574. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301087 Free PMC article.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
Cited by
-
The cold truth: torpor as a confound in studies of caloric restriction.J Comp Physiol B. 2025 Jun;195(3):263-276. doi: 10.1007/s00360-025-01616-1. Epub 2025 Jun 9. J Comp Physiol B. 2025. PMID: 40488879 Free PMC article. Review.
-
How extreme lethargy can promote healthy ageing.Nature. 2025 Mar;639(8055):549. doi: 10.1038/d41586-025-00707-x. Nature. 2025. PMID: 40087390 No abstract available.
-
N-acetylcarnosine attenuates age-associated declines in multi-organ systems to improve survival.bioRxiv [Preprint]. 2025 Aug 25:2025.08.19.671148. doi: 10.1101/2025.08.19.671148. bioRxiv. 2025. PMID: 40909683 Free PMC article. Preprint.
References
-
- Morrison, S. F. & Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol.81, 285–308 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

