Ketogenic diets and β-hydroxybutyrate in the prevention and treatment of diabetic kidney disease: current progress and future perspectives
- PMID: 40055596
- PMCID: PMC11887203
- DOI: 10.1186/s12882-025-04019-0
Ketogenic diets and β-hydroxybutyrate in the prevention and treatment of diabetic kidney disease: current progress and future perspectives
Abstract
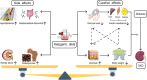
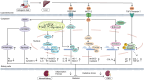
Diabetic kidney disease (DKD) is the main cause of end-stage renal disease. Ketogenic diets (KD) is a high-fat, low-carbohydrate diet. KD produces ketone bodies to supplement energy in the case of insufficient glucose in the body. β-Hydroxybutyrate (BHB) is the main component of ketone bodies. BHB serves as "ancillary fuel" substituting (but also inducing) anti-oxidative, anti-inflammatory, and cardio-protective features by binding to several target proteins, including histone acylation modification, or G protein-coupled receptors (GPCRs). KD have been used to treat epilepsy, obesity, type-2 diabetes mellitus, polycystic ovary syndrome, cancers, and other diseases. According to recent research, KD and the induced BHB delay DKD progression by improving the metabolism of glucose and lipids, regulating autophagy, as well as alleviating inflammation, oxidative stress and fibrosis. However, due to some side-effects, the role and mechanism of action of KD and BHB in the prevention and treatment of DKD are controversial. This review focuses on recent progress in the research of KD and BHB in clinical and preclinical studies of DKD, and provides new perspectives for DKD treatment.
Keywords: Diabetic kidney disease (DKD); Ketogenic diets (KD); β-hydroxybutyrate (BHB).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney.Mol Metab. 2022 Nov;65:101578. doi: 10.1016/j.molmet.2022.101578. Epub 2022 Aug 20. Mol Metab. 2022. PMID: 35995402 Free PMC article.
-
β-Hydroxybutyrate as an epigenetic modifier: Underlying mechanisms and implications.Heliyon. 2023 Oct 17;9(11):e21098. doi: 10.1016/j.heliyon.2023.e21098. eCollection 2023 Nov. Heliyon. 2023. PMID: 37928021 Free PMC article. Review.
-
Fasting and diurnal blood ketonemia and glycemia responses to a six-week, energy-controlled ketogenic diet, supplemented with racemic R/S-BHB salts.Clin Nutr ESPEN. 2023 Apr;54:277-287. doi: 10.1016/j.clnesp.2023.01.030. Epub 2023 Feb 4. Clin Nutr ESPEN. 2023. PMID: 36963874 Clinical Trial.
-
Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach.Nutrients. 2025 Apr 4;17(7):1268. doi: 10.3390/nu17071268. Nutrients. 2025. PMID: 40219025 Free PMC article. Review.
-
Dapagliflozin improves diabetic kidney disease by inhibiting ferroptosis through β-hydroxybutyrate production.Ren Fail. 2025 Dec;47(1):2438857. doi: 10.1080/0886022X.2024.2438857. Epub 2025 Jan 2. Ren Fail. 2025. PMID: 39746795 Free PMC article.
Cited by
-
Lipoprotein(a) and Effects of Diet: Time for Reassessment.Nutrients. 2025 May 19;17(10):1714. doi: 10.3390/nu17101714. Nutrients. 2025. PMID: 40431454 Free PMC article. Review.
References
-
- Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. The Lancet. 2023;402(10397):203–34. 10.1016/S0140-6736(23)01301-6. - PMC - PubMed
-
- Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434–49. 10.1111/1753-0407.12521. - PubMed
-
- Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15. 10.1111/dom.14007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFS0617/the Key Research and Development Program of Science and Technology Department of Sichuan Province
- 2022YFS0612/the Key Research and Development Program of Science and Technology Department of Sichuan Province
- 82170834/grants from the National Natural Science Foundation of China
- Z-2017-26-2202-4/the China International medical foundation
- 2020LZXNYDP02/the Office of Science Technology and Talent Work of Luzhou
LinkOut - more resources
Full Text Sources
Medical

