JAK/STAT signaling as a key regulator of ferroptosis: mechanisms and therapeutic potentials in cancer and diseases
- PMID: 40055704
- PMCID: PMC11889932
- DOI: 10.1186/s12935-025-03681-6
JAK/STAT signaling as a key regulator of ferroptosis: mechanisms and therapeutic potentials in cancer and diseases
Abstract
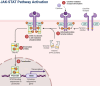
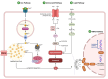
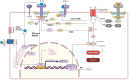
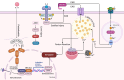
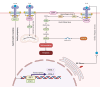
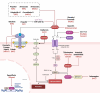
Ferroptosis is a distinct form of regulated cell death characterized by iron-dependent lipid peroxidation, playing a critical role in various diseases, including cancer, neurodegeneration, and tissue damage. This study reviews the intricate relationship between ferroptosis and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, highlighting its regulatory functions across multiple biological processes. Dysregulation of the JAK/STAT pathway is implicated in promoting or inhibiting ferroptosis, depending on the context. JAK2 promotes ferroptosis by activating STAT proteins, modulating the expression of key regulators like SLC7A11 and GPX4, and influencing iron homeostasis through pathways such as ferritinophagy and hepcidin regulation. STAT1 activation primarily enhances ferroptosis through the suppression of cystine-glutamate antiporter (System Xc-), leading to glutathione depletion and lipid peroxidation, contributing to cell death in conditions like Sjogren's syndrome and age-related macular degeneration. In contrast, STAT3 plays a protective role by upregulating SLC7A11 and GPX4, which inhibits ferroptosis and promotes cell survival, particularly in cancers such as hepatocellular carcinoma, prostate cancer, and renal cell carcinoma. This study also discusses STAT6's involvement in ferroptosis suppression in diseases like asthma and lung injury by regulating antioxidant defenses. Furthermore, the review explores potential therapeutic strategies targeting the JAK/STAT pathway to manipulate ferroptosis for disease treatment. In cancer therapy, modulating this pathway can enhance the effectiveness of ferroptosis inducers, offering promising avenues to overcome drug resistance. Additionally, the interplay between ferroptosis and JAK/STAT signaling in immune responses, oxidative stress, and lipid metabolism underscores its significance in disease progression and therapeutic intervention. By exploring these mechanisms, this study provides insights into the development of novel treatments targeting ferroptosis through JAK/STAT modulation, with implications for cancer, inflammatory diseases, and neurodegenerative conditions.
Keywords: Ferroptosis; JAK/STAT signaling; JAK2; STAT1; STAT3; STAT6; Therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Informed consent to consent for publication: Not applicable. Generative AI and AI-assisted technologies in the writing process: During the preparation of this work, the authors used ChatGPT by OpenAI to improve paper readability. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the publication’s content. Competing interests: The authors declare no competing interests.
Figures
References
-
- Pang Q, Tang Z, Luo L. The crosstalk between oncogenic signaling and ferroptosis in cancer. Crit Rev Oncol Hematol. 2024;197:104349. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

