Comprehensive Multiomic Analysis Reveals Metabolic Reprogramming Underlying Human Fontan-Associated Liver Disease
- PMID: 40055870
- PMCID: PMC12132697
- DOI: 10.1161/JAHA.124.039201
Comprehensive Multiomic Analysis Reveals Metabolic Reprogramming Underlying Human Fontan-Associated Liver Disease
Abstract
Background: The Fontan operation is the current standard of care for single-ventricle congenital heart disease. Almost all patients with Fontan operation develop liver fibrosis at a young age, known as Fontan-associated liver disease (FALD). The pathogenesis and mechanisms underlying FALD remain little understood, and there are no effective therapies. We aimed to present a comprehensive multiomic analysis of human FALD, revealing the fundamental biology and pathogenesis of FALD.
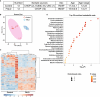
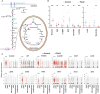
Methods and results: We recently generated a single-cell transcriptomic and epigenomic atlas of human FALD using single-nucleus multiomic RNA sequencing and assay for transposase-accessible chromatin using sequencing, which uncovered substantial metabolic reprogramming. Here, we applied liquid chromatography-mass spectrometry-based untargeted metabolomics to unveil the metabolomic landscape of human FALD, using liver samples/biopsies from age- and sex-matched donors and patients with FALD (n=12 per group). Results were integrated with liver single-nucleus multiomic RNA sequencing and assay for transposase-accessible chromatin using sequencing and serum metabolomics data to present a comprehensive multiomic atlas of FALD.We discovered significant metabolic abnormalities in livers of adolescent patients with Fontan circulation, particularly amino acid metabolism, peroxisomal fatty acid oxidation, cytochrome P450 system, glycolysis, tricarboxylic acid cycle, ketone body metabolism, and bile acid metabolism. Integrated analyses with liver single-nucleus multiomic RNA sequencing and assay for transposase-accessible chromatin using sequencing results unveiled potential underlying mechanisms of these metabolic changes. Comparison with serum metabolomics data indicate that liver metabolic reprogramming contributes to circulatory metabolomic changes in FALD. Furthermore, comparison with metabolomics data of human metabolic dysfunction-associated fatty liver disease and metabolic dysfunction-associated steatohepatitis highlighted dysregulated amino acid metabolism as a common metabolic abnormality.
Conclusions: Our comprehensive multiomic analyses reveal new insights into the fundamental biology and pathogenesis mechanisms of human FALD.
Keywords: Fontan‐associated liver disease (FALD); amino acid metabolism; metabolic reprogramming; metabolomics; multiomics.
Conflict of interest statement
Dr Rychik is a consultant for NUVO Cares. The remaining authors have no disclosures to report.
Figures




Similar articles
-
Single-cell multiomics guided mechanistic understanding of Fontan-associated liver disease.Sci Transl Med. 2024 Apr 24;16(744):eadk6213. doi: 10.1126/scitranslmed.adk6213. Epub 2024 Apr 24. Sci Transl Med. 2024. PMID: 38657025 Free PMC article.
-
Associated Factors of Liver Disease After Fontan Operation in Relation to Ultrasound Liver Elastography.Pediatr Cardiol. 2020 Dec;41(8):1639-1644. doi: 10.1007/s00246-020-02422-y. Epub 2020 Aug 1. Pediatr Cardiol. 2020. PMID: 32740670
-
Assessing hepatic impairment in Fontan-associated liver disease using the HepQuant SHUNT test.Congenit Heart Dis. 2019 Nov;14(6):978-986. doi: 10.1111/chd.12831. Epub 2019 Aug 1. Congenit Heart Dis. 2019. PMID: 31369200 Free PMC article.
-
Imaging of Fontan-associated liver disease.Pediatr Radiol. 2020 Oct;50(11):1528-1541. doi: 10.1007/s00247-020-04776-0. Epub 2020 Aug 18. Pediatr Radiol. 2020. PMID: 32809067 Review.
-
Fontan-Associated Liver Disease: Proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC.J Am Coll Cardiol. 2017 Dec 26;70(25):3173-3194. doi: 10.1016/j.jacc.2017.10.045. J Am Coll Cardiol. 2017. PMID: 29268929 Review.
Cited by
-
Overcoming borders: International cooperation in re-use and re-interpretation of omics data in Fontan circulation.Int J Cardiol Congenit Heart Dis. 2025 May 5;20:100590. doi: 10.1016/j.ijcchd.2025.100590. eCollection 2025 Jun. Int J Cardiol Congenit Heart Dis. 2025. PMID: 40475704 Free PMC article. No abstract available.
References
-
- Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia TY, Hsu DT, Kovacs AH, McCrindle BW, et al. Evaluation and management of the child and adult with Fontan Circulation: a scientific statement from the American Heart Association. Circulation. 2019;140:CIR0000000000000696. doi: 10.1161/CIR.0000000000000696 - DOI - PubMed
-
- Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, Rome JJ, Rand EB, Russo P, et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.004809 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

