Transcriptomic changes in oxidative stress, immunity, and cancer pathways caused by cannabis vapor on alveolar epithelial cells
- PMID: 40056285
- PMCID: PMC11890392
- DOI: 10.1007/s10565-025-09997-3
Transcriptomic changes in oxidative stress, immunity, and cancer pathways caused by cannabis vapor on alveolar epithelial cells
Abstract
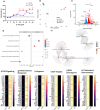
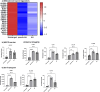
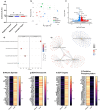
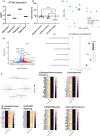
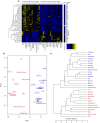
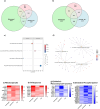
As legalization of cannabis increases worldwide, vaping cannabis is gaining popularity due to the belief that it is less harmful than smoking cannabis. However, the safety of cannabis vaping remains untested. To address this, we developed a physiologically relevant method for in vitro assessment of cannabis vapor on alveolar epithelial cell cultures. We compared the transcriptional response in three in vitro models of cannabis vapor exposure using A549 epithelial cells in submerged culture, pseudo-air liquid interface (ALI) culture, and ALI culture coupled with the expoCube™ advanced exposure system. Baseline gene expression in ALI-maintained A549 cells showed higher expression of type 2 alveolar epithelial (AEC2) genes related to surfactant production, ion movement, and barrier integrity. Acute exposure to cannabis vapor significantly affected gene expression in AEC2 cells belonging to pathways related to cancer, oxidative stress, and the immune response without being associated with a DNA damage response. This study identifies potential risks of cannabis vaping and underscores the need for further exploration into its respiratory health implications.
Keywords: Air–liquid interface; Cannabis vapor; Lung cancer; New approach methods.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: PG is employed at SCIREQ- Scientific Respiratory Equipment, Inc., ETW completed an internship at SCIREQ- Scientific Respiratory Equipment, Inc., through the MITACS Accelerate Program. The other authors have no conflicts to disclose.
Figures
Similar articles
-
Cannabis vaping elicits transcriptomic and metabolomic changes involved in inflammatory, oxidative stress, and cancer pathways in human bronchial epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2025 Mar 1;328(3):L478-L496. doi: 10.1152/ajplung.00131.2024. Epub 2025 Jan 17. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39823205
-
24-Dehydrocholesterol Reductase alleviates oxidative damage-induced apoptosis in alveolar epithelial cells via regulating Phosphatidylinositol-3-Kinase/Protein Kinase B activation.Bioengineered. 2022 Jan;13(1):155-163. doi: 10.1080/21655979.2021.2011634. Bioengineered. 2022. PMID: 34949154 Free PMC article.
-
Pro-inflammatory and genotoxic responses by metal oxide nanomaterials in alveolar epithelial cells and macrophages in submerged condition and air-liquid interface: An in vitro-in vivo correlation study.Toxicol In Vitro. 2024 Oct;100:105897. doi: 10.1016/j.tiv.2024.105897. Epub 2024 Jul 25. Toxicol In Vitro. 2024. PMID: 39025158
-
Overview on biological implications of metal oxide nanoparticle exposure to human alveolar A549 cell line.Nanotoxicology. 2017 Aug;11(6):713-724. doi: 10.1080/17435390.2017.1366574. Epub 2017 Aug 23. Nanotoxicology. 2017. PMID: 28830283 Review.
-
The role of miRNAs in alveolar epithelial cells in emphysema.Biomed Pharmacother. 2021 Nov;143:112216. doi: 10.1016/j.biopha.2021.112216. Epub 2021 Sep 27. Biomed Pharmacother. 2021. PMID: 34649347 Free PMC article. Review.
References
-
- Abaji C, Cousineau I, Belmaaza A. BRCA2 regulates homologous recombination in response to DNA damage: implications for genome stability and carcinogenesis. Cancer Res. 2005;65:4117–25. 10.1158/0008-5472.Can-04-3071. - PubMed
-
- Albert Stuart R, Gary KH. Chromothripsis and epigenomics complete causality criteria for cannabis- and addiction-connected carcinogenicity, congenital toxicity and heritable genotoxicity. Mutation Research/fundamental and Molecular Mechanisms of Mutagenesis. 2016;789:15–25. 10.1016/j.mrfmmm.2016.05.002. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

