Raised intracranial pressure alters cortical vascular function and cephalic allodynia
- PMID: 40056451
- PMCID: PMC12129729
- DOI: 10.1093/brain/awae415
Raised intracranial pressure alters cortical vascular function and cephalic allodynia
Abstract
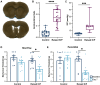
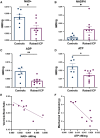
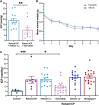
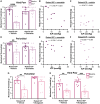
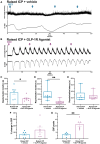
Raised intracranial pressure (ICP) is associated with altered cerebral haemodynamics and cephalic pain. The relationship between the algetic response and cortical neurovascular changes in raised ICP is unclear. This study aimed to evaluate this relationship and determine whether lowering ICP (using a glucagon-like peptide-1 receptor agonist) could ameliorate the algetic response. We also sought to explore the role of calcitonin gene-related peptide in cephalic pain driven by raised ICP by inhibiting calcitonin gene-related peptide signalling and quantifying changes in the algetic response. In a rat model of raised ICP, created by intracisternal kaolin injection, mechanical thresholds were measured alongside steady-state potential and cerebral blood flow responses to spreading depolarization. Nuclear magnetic resonance spectroscopy evaluated energetic substrates in animals with raised ICP ex vivo. The glucagon-like peptide-1 receptor (GLP-1R) agonist exenatide and the calcitonin gene-related peptide receptor (CGRP-R) antagonist olcegepant were injected daily, and measurements were repeated. Kaolin increased ICP [median (range) 15.96 (8.97) mmHg, n = 8] versus controls [6.02 (1.79) mmHg, n = 6, P = 0.0007]. Animals with raised ICP exhibited reduced mechanical thresholds [mean (standard deviation) hind paw baseline: 5.78 (2.81) g, Day 7: 3.34 (2.22) g, P < 0.001; periorbital baseline: 6.13 (2.07) g, Day 7: 2.35 (1.91) g, n = 12, P < 0.001]. Depolarization and repolarization durations were increased [depolarization, raised ICP: 108.81 (222.12) s, n = 11, controls: 37.54 (108.38) s, n = 9, P = 0.038; repolarization, raised ICP: 1824.26 (3499.54) s, n = 12, controls: 86.96 (140.05) s, n = 9, P < 0.0001]. Cerebral blood flow change was also reduced [85.55 (30.84)%, n = 9] compared with controls [217.64 (37.70)%, n = 8, P < 0.0001]. Substrates for cellular energetics (ADP, ATP and NAD+) were depleted in rodent brains with raised ICP (P = 0.009, P = 0.018 and P = 0.011, respectively). Exenatide significantly lowered ICP [exenatide: 9.74 (6.09) mmHg, n = 19, vehicle: 18.27 (6.67) mmHg, n = 16, P = 0.004] and rescued changes in mechanical withdrawal. Exenatide recovered characteristic spreading depolarization responses [depolarization duration, exenatide: 56.46 (25.10) s, n = 7, vehicle: 115.98 (58.80) s, n = 6, P = 0.033; repolarization duration, exenatide: 177.55 (562.88) s, n = 7, vehicle: 800.85 (1988.67) s, n = 6, P = 0.002]. In the setting of raised ICP, olcegepant prevented changes in periorbital mechanical thresholds. We conclude that raised ICP disrupted the cortical neurovascular responses, reduced algetic thresholds and depleted crucial energetic substrates. Exenatide reduced ICP, improving algetic thresholds and cortical neurovascular changes. Importantly, olcegepant alleviated the cerebral algesia, suggesting a role for calcitonin gene-related peptide in driving pain responses in elevated ICP. These studies support the rationale that reducing ICP improves cephalic pain in conditions of raised ICP. Furthermore, the data suggest that headache pain in diseases associated with raised ICP could be ameliorated therapeutically though blockade of the calcitonin gene-related peptide pathway.
Keywords: calcitonin gene-related peptide receptor antagonist; exenatide; glucagon-like peptide receptor agonist; olcegepant.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
O.G. reports scientific consultancy fees from Invex Therapeutics 2020 that were outside the work in this article. A.J.S. reports previous personal fees from Invex therapeutics during her role as director with stock holdings (2019–2022); other fees for advisory boards with Allergan, Novartis, Cheisi and Amgen outside the submitted work. All other authors report no competing interests.
Figures


References
-
- Czosnyka M, Smielewski P, Piechnik S, et al. Hemodynamic characterization of intracranial pressure plateau waves in head-injury patients. J Neurosurg. 1999;91:11–19. - PubMed
-
- Kelly DF, Martin NA, Kordestani R, et al. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86:633–641. - PubMed
-
- Walker WC, Seel RT, Curtiss G, Warden DL. Headache after moderate and severe traumatic brain injury: A longitudinal analysis. Arch Phys Med Rehabil. 2005;86:1793–1800. - PubMed
MeSH terms
Substances
Grants and funding
- MR/P006264/1/Almirall
- NIHR-CS-011-028/NIHR
- Biomedical Science
- National Institute for Health Research
- 104612/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- Novartis
- Amgen
- Teva
- Pfizer
- MR/N013700/1/Migraine Trust UK Fellowship
- WT_/Wellcome Trust/United Kingdom
- MR/K015184/1/MRC_/Medical Research Council/United Kingdom
- Migraine Trust UK Fellowship
LinkOut - more resources
Full Text Sources
Research Materials

