Data Interoperability in COVID-19 Vaccine Trials: Methodological Approach in the VACCELERATE Project
- PMID: 40056469
- PMCID: PMC11928774
- DOI: 10.2196/65590
Data Interoperability in COVID-19 Vaccine Trials: Methodological Approach in the VACCELERATE Project
Abstract
Background: Data standards are not only key to making data processing efficient but also fundamental to ensuring data interoperability. When clinical trial data are structured according to international standards, they become significantly easier to analyze, reducing the efforts required for data cleaning, preprocessing, and secondary use. A common language and a shared set of expectations facilitate interoperability between systems and devices.
Objective: The main objectives of this study were to identify commonalities and differences in clinical trial metadata, protocols, and data collection systems/items within the VACCELERATE project.
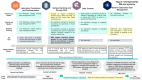
Methods: To assess the degree of interoperability achieved in the project and suggest methodological improvements, interoperable points were identified based on the core outcome areas-immunogenicity, safety, and efficacy (clinical/physiological). These points were emphasized in the development of the master protocol template and were manually compared in the following ways: (1) summaries, objectives, and end points in the protocols of 3 VACCELERATE clinical trials (EU-COVAT-1_AGED, EU-COVAT-2_BOOSTAVAC, and EU-COVPT-1_CoVacc) against the master protocol template; (2) metadata of all 3 clinical trials; and (3) evaluations from a questionnaire survey regarding differences in data management systems and structures that enabled data exchange within the VACCELERATE network.
Results: The noncommonalities identified in the protocols and metadata were attributed to differences in populations, variations in protocol design, and vaccination patterns. The detailed metadata released for all 3 vaccine trials were clearly structured using internal standards, terminology, and the general approach of Clinical Data Acquisition Standards Harmonisation (CDASH) for data collection (eg, on electronic case report forms). VACCELERATE benefited significantly from the selection of the Clinical Trials Centre Cologne as the sole data management provider. With system database development coordinated by a single individual and no need for coordination among different trial units, a high degree of uniformity was achieved automatically. The harmonized transfer of data to all sites, using well-established methods, enabled quick exchanges and provided a relatively secure means of data transfer.
Conclusions: This study demonstrated that using master protocols can significantly enhance trial operational efficiency and data interoperability, provided that similar infrastructure and data management procedures are adopted across multiple trials. To further improve data interoperability and facilitate interpretation and analysis, shared data should be structured, described, formatted, and stored using widely recognized data and metadata standards.
Trial registration: EudraCT 2021-004526-29; https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-004526-29/DE/; 2021-004889-35; https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2021-004889-35; and 2021-004526-29; https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2021-004526-29.
Keywords: adult; clinical trials; data management; harmonization; interoperability; metadata; pediatric; protocol; standards; systems.
©Salma Malik, Zoi Pana Dorothea, Christos D Argyropoulos, Sophia Themistocleous, Alan J Macken, Olena Valdenmaiier, Frank Scheckenbach, Elena Bardach, Andrea Pfeiffer, Katherine Loens, Jordi Cano Ochando, Oliver A Cornely, Jacques Demotes-Mainard, Sergio Contrino, Gerd Felder. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 07.03.2025.
Conflict of interest statement
Conflicts of Interest: OAC reports grants or contracts from iMi, iHi, DFG, BMBF, Cidara, DZIF, EU-DG RTD, F2G, Gilead, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; consulting fees from Abbvie, AiCuris, Basilea, Biocon, Boston Strategic Partners, Cidara, Elion Therapeutics, Gilead, GSK, IQVIA, Janssen, Matinas, MedPace, Menarini, Melinta, Molecular Partners, MSG-ERC, Mundipharma, Noxxon, Octapharma, Pardes, Partner Therapeutics, Pfizer, PSI, Scynexis, Seres, Seqirus, Shionogi, The Prime Meridian Group; speaker and lecture honoraria from Abbott, Abbvie, Akademie für Infektionsmedizin, Al-Jazeera Pharmaceuticals/Hikma, amedes, AstraZeneca, Deutscher Ärzteverlag, Gilead, GSK, Grupo Biotoscana/United Medical/Knight, Ipsen Pharma, Medscape/WebMD, MedUpdate, MSD, Moderna, Mundipharma, Noscendo, Paul-Martini-Stiftung, Pfizer, Sandoz, Seqirus, Shionogi, streamedup!, Touch Independent, Vitis; payment for expert testimony Cidara; and participation on a DRC, DSMB, DMC, Advisory Board for AstraZeneca, Cidara, IQVIA, Janssen, MedPace, Melinta, PSI, Pulmocide, Vedanta Biosciences. All other authors report no conflicting interests.
Figures





References
-
- Interoperability in healthcare. Healthcare Information and Management Systems Society. [2023-06-05]. https://www.himss.org/resources/interoperability-healthcare#Part1 .
-
- Wilkinson Mark D, Dumontier Michel, Aalbersberg I Jsbrand Jan, Appleton Gabrielle, Axton Myles, Baak Arie, Blomberg Niklas, Boiten Jan-Willem, da Silva Santos Luiz Bonino, Bourne Philip E, Bouwman Jildau, Brookes Anthony J, Clark Tim, Crosas Mercè, Dillo Ingrid, Dumon Olivier, Edmunds Scott, Evelo Chris T, Finkers Richard, Gonzalez-Beltran Alejandra, Gray Alasdair J G, Groth Paul, Goble Carole, Grethe Jeffrey S, Heringa Jaap, 't Hoen Peter A C, Hooft Rob, Kuhn Tobias, Kok Ruben, Kok Joost, Lusher Scott J, Martone Maryann E, Mons Albert, Packer Abel L, Persson Bengt, Rocca-Serra Philippe, Roos Marco, van Schaik Rene, Sansone Susanna-Assunta, Schultes Erik, Sengstag Thierry, Slater Ted, Strawn George, Swertz Morris A, Thompson Mark, van der Lei Johan, van Mulligen Erik, Velterop Jan, Waagmeester Andra, Wittenburg Peter, Wolstencroft Katherine, Zhao Jun, Mons Barend. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016 Mar 15;3:160018. doi: 10.1038/sdata.2016.18.sdata201618 - DOI - PMC - PubMed
-
- Ohmann Christian, Banzi Rita, Canham Steve, Battaglia Serena, Matei Mihaela, Ariyo Christopher, Becnel Lauren, Bierer Barbara, Bowers Sarion, Clivio Luca, Dias Monica, Druml Christiane, Faure Hélène, Fenner Martin, Galvez Jose, Ghersi Davina, Gluud Christian, Groves Trish, Houston Paul, Karam Ghassan, Kalra Dipak, Knowles Rachel L, Krleža-Jerić Karmela, Kubiak Christine, Kuchinke Wolfgang, Kush Rebecca, Lukkarinen Ari, Marques Pedro Silverio, Newbigging Andrew, O'Callaghan Jennifer, Ravaud Philippe, Schlünder Irene, Shanahan Daniel, Sitter Helmut, Spalding Dylan, Tudur-Smith Catrin, van Reusel Peter, van Veen Evert-Ben, Visser Gerben Rienk, Wilson Julia, Demotes-Mainard Jacques. Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open. 2017 Dec 14;7(12):e018647. doi: 10.1136/bmjopen-2017-018647. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=29247106 bmjopen-2017-018647 - DOI - PMC - PubMed
-
- VACCELERATE - European Corona Vaccine Trial Accelerator platform. VACCELERATE. [2023-10-26]. https://vaccelerate.eu/
-
- Salmanton-García Jon, Stewart FA, Heringer S, Koniordou M, Álvarez-Barco Elena, Argyropoulos CD, Themistocleous SC, Valle-Simón Paula, Spivak O, Součková Lenka, Merakou C, Amélia Mendonça Maria, Joanna Davis R, Maria Azzini A, Askling HH, Vene S, Van Damme P, Steinbach A, Shiamakkides G, Seidel D, Olesen OF, Noula E, Macken A, Luís Catarina, Leckler J, Launay O, Isitt C, Hellemans M, Frías-Iniesta Jesús, Di Marzo R, Carcas AJ, Boustras G, Borobia AM, Barta I, Albus K, Akova M, Ochando J, Cohen-Kandli M, Jane Cox R, Husa P, Jancoriene L, Mallon P, Marques L, Mellinghoff SC, Nauclér Pontus, Tacconelli E, Tóth Krisztina, Zaoutis TE, Zeitlinger M, Cornely OA, Pana Z. VACCELERATE volunteer registry: a European study participant database to facilitate clinical trial enrolment. Vaccine. 2022 Jul 29;40(31):4090–4097. doi: 10.1016/j.vaccine.2022.05.022. https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(22)00605-3 S0264-410X(22)00605-3 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

