Choline alleviates cognitive impairment in sleep-deprived young mice via reducing neuroinflammation and altering phospholipidomic profile
- PMID: 40056720
- PMCID: PMC11930228
- DOI: 10.1016/j.redox.2025.103578
Choline alleviates cognitive impairment in sleep-deprived young mice via reducing neuroinflammation and altering phospholipidomic profile
Abstract
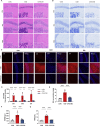
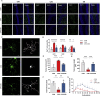
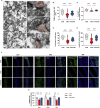
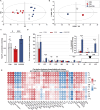
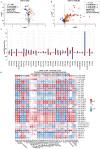
Cognitive impairment resulting from insufficient sleep poses a significant public health concern, particularly in children. The effects and mechanisms of choline on cognitive impairment caused by sleep deprivation are unknown. Chronic sleep deprivation is induced in young mice in this study, followed by feeding diet containing 11.36 g/kg choline bitartrate. Choline supplementation significantly improves spatial learning ability. Functional MRI results reveal the hippocampus as a key region affected by sleep deprivation, where choline supplementation notably preserves hippocampal structural integrity and enhanced connectivity. Additionally, choline ameliorates hippocampal pathological injury, reduces blood-brain barrier permeability and serum brain injury biomarkers. Choline also reduces inflammation and oxidative stress biomarkers, and mitigates microglial activation in the hippocampus, which preserves synaptic plasticity. A key finding is the changes of hippocampal phospholipidomic profile along with cognitive function, and a total of 313 phospholipid molecules are identified. Choline increases the levels of total phospholipid and sub-classes (particularly PC), which are strongly correlated with reduced neuroinflammation and oxidative stress biomarkers, as well as improved cognitive outcomes. Furthermore, there are similar findings in some phospholipid molecules such as PC 36:1, PC O-33:0, PC p-38:3, PE 36:3, PE p-42:4 and PS 44:12. These findings highlight that choline alleviates cognitive impairment in sleep deprivation via reducing neuroinflammation and oxidative stress as well as altering phospholipidomic profile. This study suggests that choline could develop into functional food or medicine ingredient to prevent and treat cognitive impairment by sleep disturbances, particularly children and adolescents.
Keywords: Choline; Cognitive impairment; Magnetic resonance imaging; Neuroinflammation; Phospholipidomics; Sleep deprivation.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O'Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Adams Hillard P.J. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

