Redefining radiologic responses in high-risk soft-tissue sarcomas treated with neoadjuvant chemotherapy: final results of ISG-STS 1001, a randomized clinical trial
- PMID: 40056850
- PMCID: PMC11930716
- DOI: 10.1016/j.esmoop.2025.104299
Redefining radiologic responses in high-risk soft-tissue sarcomas treated with neoadjuvant chemotherapy: final results of ISG-STS 1001, a randomized clinical trial
Abstract
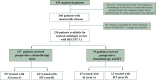
Background: We report the results of the pre-planned secondary analysis of radiologic responses (RRs) of ISG-STS 1001, a randomized trial comparing anthracycline + ifosfamide (AI) versus histology-tailored (HT) neoadjuvant chemotherapy for primary localized high-risk soft-tissue sarcomas of the extremities and trunk wall.
Patients and methods: Patients with undifferentiated pleomorphic sarcoma (UPS), leiomyosarcoma (LMS), malignant peripheral nerve sheath tumor, synovial sarcoma or myxoid liposarcoma (MLPS) were randomized, whereas patients with myxofibrosarcoma, pleomorphic liposarcoma, pleomorphic rhabdomyosarcoma or unclassified sarcoma were included in the observational arm (O) and treated with AI. Patients with UPS, LMS or MLPS needing concurrent preoperative radiotherapy were included in O. We evaluated associations between: disease-free survival (DFS)/overall survival (OS) and centrally reviewed RR, assessed with RECIST 1.1 and as percent dimensional variation (D; both dichotomized and continuous); DFS/OS and histology; RR and histology.
Results: Four hundred and thirty-five patients were included (287 randomized, 148 observed). The analysis of RRs comprised 236 patients (154 randomized, 82 observed) with measurable disease and available for central review. RECIST best responses were: 28 (11.9%) partial response (PR), 195 (82.6%) stable disease (SD), 13 (5.5%) progressive disease (PD). RECIST significantly correlated with DFS [PD versus PR: hazard ratio (HR) 8.18, 95% confidence interval (CI) 2.96-22.58; SD versus PR: HR 2.96, 95% CI 1.30-6.75] and OS (PD versus PR: HR 12.61, 95% CI 3.40-46.84; SD versus PR: HR 4.24, 95% CI 1.34-13.47). The median value of D was -1.6%. Patients with D >-1.6% had worse clinical outcomes than those with D <-1.6% (DFS: HR 1.73, 95% CI 1.19-2.50; OS: HR 1.86, 95% CI 1.21-2.86). D in continuous scale inversely correlated with DFS (HR 1.53, 95% CI 1.25-1.87) and OS (HR 1.78, 95% CI 1.41-2.25).
Conclusions: These results confirm the prognostic value of RRs as per RECIST and D and demonstrate that any variation in size predicts the proportional efficacy of treatment.
Keywords: RECIST; neoadjuvant chemotherapy; prognosis; randomized clinical trial; sarcoma; tumor response.
Copyright © 2025. Published by Elsevier Ltd.
Figures



References
-
- Gronchi A., Frustaci S., Mercuri M., et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30(8):850–856. - PubMed
-
- Gronchi A., Stacchiotti S., Verderio P., et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol. 2016;27(12):2283–2288. - PubMed
-
- Gronchi A., Ferrari S., Quagliuolo V., et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–822. - PubMed
-
- Gronchi A., Palmerini E., Quagliuolo V., et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) sarcoma groups. J Clin Oncol. 2020;38(19):2178–2186. - PubMed
-
- Palassini E., Ferrari S., Verderio P., et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian sarcoma group/grupo español de investigación en sarcomas randomized clinical trial: three versus five cycles of full-dose epirubicin plus ifosfamide. J Clin Oncol. 2015;33(31):3628–3634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

