A circadian and app-based personalized lighting intervention for the reduction of cancer-related fatigue
- PMID: 40056908
- PMCID: PMC11970396
- DOI: 10.1016/j.xcrm.2025.102001
A circadian and app-based personalized lighting intervention for the reduction of cancer-related fatigue
Abstract
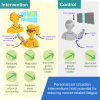
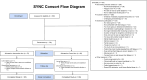
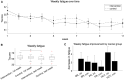
Lighting interventions can mitigate fatigue by promoting circadian rhythmicity. We test whether individualized, wearable-based lighting interventions delivered via a mobile app reduce cancer-related fatigue in a randomized controlled trial with 138 breast cancer, prostate cancer, and hematopoietic stem cell transplant patients. Participants are randomized to tailored lighting intervention or control. The primary endpoint is PROMIS fatigue 4a at trial end, with secondary endpoints including change in daily fatigue, sleep, anxiety, depression, physical function, and overall health. Fatigue T-scores at week 11 do not differ between groups but decrease significantly from week 1 to week 11 (3.07 points, p = 0.001) in the intervention group, with a significant final-week treatment effect (p = 0.014). Daily fatigue, anxiety, sleep disturbance, and physical function improve within intervention. Further studies are needed to see if these results generalize in broader cancer care. The trial is registered at ClinicalTrials.gov (trial registration number: NCT04827446).
Keywords: cancer; cancer-related fatigue; circadian rhythm; lighting interventions; mathematical models; personalized medicine; precision medicine; wearable devices.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests O.W. has given talks at Unilever events and received honorariums/travel expenses; she has also done consulting for Gideon Health. She is the CEO of Arcascope, a company that makes circadian rhythm software. D.B.F. is the CSO of Arcascope. D.B.F. and the University of Michigan are part owners of Arcascope. N.L.H. has received institutional support for the conduct of a clinical trial (Blue Note Therapeutics), receives royalties from UpToDate, and has consulted for AstraZeneca and Myovant Sciences. Z.R.R. has received institutional support from AstraZeneca and consulted for AstraZeneca and Johnson and Johnson.
Figures


References
-
- Hofman M., Ryan J.L., Figueroa-Moseley C.D., Jean-Pierre P., Morrow G.R. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12:4–10. - PubMed
-
- Stone P.C., Minton O. Cancer-related fatigue. Eur. J. Cancer. 2008;44:1097–1104. - PubMed
-
- Ryan J.L., Carroll J.K., Ryan E.P., Mustian K.M., Fiscella K., Morrow G.R. Mechanisms of cancer-related fatigue. Oncologist. 2007;12:22–34. - PubMed
-
- Roscoe J.A., Morrow G.R., Hickok J.T., Bushunow P., Matteson S., Rakita D., Andrews P.L.R. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support. Care Cancer. 2002;10:329–336. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

