Engineered human myogenic cells in hydrogels generate innervated vascularized myofibers within dystrophic mouse muscle on long-term engraftment
- PMID: 40056909
- PMCID: PMC11970389
- DOI: 10.1016/j.xcrm.2025.102019
Engineered human myogenic cells in hydrogels generate innervated vascularized myofibers within dystrophic mouse muscle on long-term engraftment
Abstract
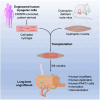
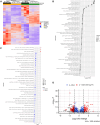
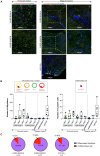
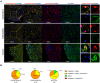
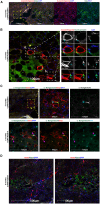
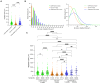
Transplantation of human myogenic progenitor cells (MPCs) is a promising therapeutic strategy for treating muscle-wasting diseases, e.g., Duchenne muscular dystrophy (DMD). To increase engraftment efficiency of donor stem cells, modulation of host muscles is required, significantly limiting their clinical translation. Here, we develop a clinically relevant transplantation strategy synergizing hydrogel-mediated delivery and engineered human MPCs generated from CRISPR-corrected DMD patient-derived pluripotent stem cells. We demonstrate that donor-derived human myofibers produce full-length dystrophin at 4 weeks and 5-6 months (long-term) after transplantation in the unmodulated muscles of the dystrophin-deficient mouse model of DMD. Remarkably, human myofibers are innervated by mouse motor neurons forming neuromuscular junctions and supported by vascularization after long-term engraftment in dystrophic mice. PAX7+ cells of human origin populate the satellite cell niche. There was no evidence of tumorigenesis in mice engrafted with hydrogel-encapsulated human MPCs. Our results provide a proof of concept in developing hydrogel-based cell therapy for muscle-wasting diseases.
Keywords: CRISPR; Duchenne muscular dystrophy; biomaterials; dystrophin; hydrogels; innervation; regenerative medicine; stem cells; vascularization; xenoengraftment.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.-Y.L. was the Principal Investigator in a research project funded by Pfizer. O.P. is a co-founder and shareholder of Somite Therapeutics.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

