CD38 in the pathobiology of cutaneous T-cell lymphoma and the potential for combination therapeutic intervention
- PMID: 40057636
- PMCID: PMC12055602
- DOI: 10.1038/s41375-025-02551-4
CD38 in the pathobiology of cutaneous T-cell lymphoma and the potential for combination therapeutic intervention
Abstract
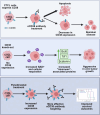
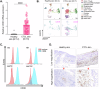
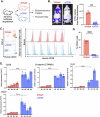
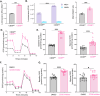
Cutaneous T-Cell Lymphoma (CTCL) is a non-Hodgkin's lymphoma involving malignant skin-homing T-cells, characterized by variable severity and limited treatment options. Our study shows that patient samples and derived cell lines express CD38 on CTCL cells, and αCD38 antibodies effectively target CD38 in a mouse model. In vivo αCD38 antibody treatment led to the loss of CD38 expression in residual tumor cells, highlighting the need for innovative strategies to improve CTCL outcomes despite the CD38 loss in residual tumor cells. To investigate the role of CD38 in CTCL pathology, we used CRISPR-Cas9 to create CD38-deficient (CD38KO) CTCL cells. These CD38KO cells showed higher expression of oncogenes B-catenin, TCF7, and BCL6, along with reduced migration. Elevated NAD+ levels in CD38KO cells increased cellular respiration after CD38 inhibition in CD38WT cells. In vivo, CD38KO cell transplants led to more aggressive tumors, likely due to elevated β-catenin, Bcl6, and Tcf-1 signaling. Prior research in multiple myeloma showed αCD38 antibody efficacy relies on CD38 expression. We discovered that panobinostat, a histone deacetylase inhibitor, increased surface CD38 expression in CTCL cells dose-dependently. Combining panobinostat with αCD38 antibody in a CTCL mouse model significantly improved survival compared to the antibody alone, underscoring CD38's therapeutic potential in CTCL. CD38 is expressed in CTCL cells and can be targeted with αCD38 antibody. αCD38 antibody treatment leads to a significant reduction in CTCL cells, while residual cells lose CD38 expression. Knocking out CD38 from CTCL cells leads to increases in intracellular NAD+ and increased cellular respiration. Additionally, CD38KO cells have increased protein levels of β-catenin, Tcf1 (encoded by TCF7), and Bcl6. CD38KO CTCL cells grow more aggressively in vivo than CD38WT CTCL cells. Treating CTCL cells with panobinostat increases CD38 expression. A dual combination treatment of panobinostat and αCD38 antibody in a mouse model of CTCL improved survival outcomes compared to αCD38 antibody treatment alone. (Figure made with Biorender.com).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All experiments were performed in accordance with relevant guidelines and regulations. All animal experiments were conducted with Institutional Animal Care and Use Committee (ID 02234) approval. Studies including human subjects or samples were approved by an Institutional Review Board (IRB 17D.083) and conducted in accordance with the Declaration of Helsinki with informed consent obtained from all subjects.
Figures



References
-
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–9. - PubMed
-
- Duarte RF, Boumendil A, Onida F, Gabriel I, Arranz R, Arcese W, et al. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol. 2014;32:3347–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

