IL-19 as a promising theranostic target to reprogram the glioblastoma immunosuppressive microenvironment
- PMID: 40057744
- PMCID: PMC11889942
- DOI: 10.1186/s12929-025-01126-w
IL-19 as a promising theranostic target to reprogram the glioblastoma immunosuppressive microenvironment
Abstract
Background: Glioblastoma multiforme (GBM) is an aggressive brain tumor with chemoresistant, immunosuppressive, and invasive properties. Despite standard therapies, including surgery, radiotherapy, and temozolomide (TMZ) chemotherapy, tumors inevitably recur in the peritumoral region. Targeting GBM-mediated immunosuppressive and invasive properties is a promising strategy to improve clinical outcomes.
Methods: We utilized clinical and genomic data from the Taiwan GBM cohort and The Cancer Genome Atlas (TCGA) to analyze RNA sequencing data from patient tumor samples, determining the association of interleukin-19 (Il-19) expression with survival and immunosuppressive activity. Gene set enrichment analysis (GSEA) was performed to assess the relationship between the enrichment levels of immune subsets and Il-19 expression level, and Ingenuity Pathway Analysis (IPA) was used to predict immune responses. Cytokine array and single-cell RNA sequencing were used to examine the effects of IL-19 blockade on tumor immune microenvironment, including tumor-infiltrating leukocyte profiles, differentiation and immunosuppressive genes expression in tumor associated macrophages (TAM). CRISPR Il-19-/- cell lines and Il-19-/- mice were used to examine the role of IL-19 in tumor invasion and M2-like macrophage-mediated immunosuppression. Additionally, we developed novel cholesterol-polyethylene glycol-superparamagnetic iron oxide-IL-19 antibody nanoparticles (CHOL-PEG-SPIO-IL-19), characterized them using dynamic light scattering and transmission electron microscopy, Fourier-Transform Infrared spectroscopy, prussian blue assay, and conducted in vivo magnetic resonance imaging (MRI) in a human glioblastoma stem cell-derived GBM animal model.
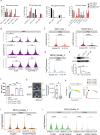
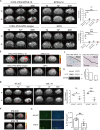
Result: Genomic screening and IPA analysis identified IL-19 as a predicted immunosuppressive cytokine in the peritumoral region, associated with poor survival in patients with GBM. Blocking IL-19 significantly inhibited tumor progression of both TMZ-sensitive (TMZ-S) and TMZ-resistant (TMZ-R) GBM-bearing mice, and modulated the immune response within the GBM microenvironment. Single-cell transcriptome analysis reveal that IL-19 antibody treatment led to a marked increase in dendritic cells and monocyte/macrophage subsets associated with interferon-gamma signaling pathways. IL-19 blockade promoted T cell activation and reprogrammed tumor-associated macrophages toward weakened pro-tumoral phenotypes with reduced Arginase 1 expression. Il19-/- M2-like bone marrow-derived macrophages with lower Arginase 1 level lost their ability to suppress CD8 T cell activation. These findings indicated that IL-19 suppression limits TAM-mediated immune suppression. Molecular studies revealed that IL-19 promotes TMZ-resistant GBM cell migration and invasion through a novel IL-19/WISP1 signaling pathway. For clinical translation, we developed a novel CHOL-PEG-SPIO-IL-19 nanoparticles to target IL-19 expression in glioblastoma tissue. MRI imaging demonstrated enhanced targeting efficiency in brain tumors, with in vivo studies showing prominent hypointense areas in T2*-weighted MRI scans of tumor-bearing mice injected with CHOL-PEG-SPIO-IL-19, highlighting nanoparticle presence in IL-19-expressing regions. Prussian blue staining further confirmed the localization of these nanoparticles in tumor tissues, verifying their potential as a diagnostic tool for detecting IL-19 expression in glioblastoma. This system offers a theranostic approach, integrating diagnostic imaging and targeted therapy for IL-19-expressing GBM.
Conclusion: IL-19 is a promising theranostic target for reversing immunosuppression and restricting the invasive activity of chemoresistant GBM cells.
Keywords: Glioblastoma; IL-19; Temozolomide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board of Taipei Medical University (no. N201603086). All animal care and experimental procedures were approved by the Ethics Committee of Taipei Medical University and conducted in strict accordance with the ARRIVE Guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Abdelfattah N, Kumar P, Wang C, Leu JS, Flynn WF, Gao R, Baskin DS, Pichumani K, Ijare OB, Wood SL, Powell SZ, Haviland DL, Parker Kerrigan BC, Lang FF, Prabhu SS, Huntoon KM, Jiang W, Kim BYS, George J, Yun K. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat Commun. 2022;13(1):767. - PMC - PubMed
-
- Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16(6):1017–28. - PubMed
-
- Bergmann N, Delbridge C, Gempt J, Feuchtinger A, Walch A, Schirmer L, Bunk W, Aschenbrenner T, Liesche-Starnecker F, Schlegel J. The intratumoral heterogeneity reflects the intertumoral subtypes of glioblastoma multiforme: a regional immunohistochemistry analysis. Front Oncol. 2020;10:494. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

