Prevalence of Mpox vaccine acceptance and hesitancy among people living with HIV: a comprehensive systematic review and meta-analysis
- PMID: 40057788
- PMCID: PMC11889782
- DOI: 10.1186/s12981-025-00726-8
Prevalence of Mpox vaccine acceptance and hesitancy among people living with HIV: a comprehensive systematic review and meta-analysis
Abstract
Background: Vaccine acceptance among People Living with HIV (PLWH) is crucial for managing and mitigating the spread of infectious diseases, including Mpox. This systematic review and meta-analysis assess the rate of vaccine acceptance for Mpox among PLWH and identify factors influencing these rates.
Methods: We searched major databases including PubMed, Embase, and Web of Science up to 30 August 2024 for observational studies involving PLWH that reported on mpox vaccine acceptance rates. A random-effects model was employed for the meta-analysis, utilizing R software version 4.4. Heterogeneity among the studies was quantified using the I² statistic, and the methodological quality of each study was assessed using a modified version of the Newcastle-Ottawa Scale.
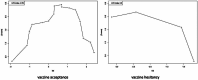
Results: Out of 1,123 articles identified, 17 studies met the inclusion criteria and included 7,248 participants. The pooled estimate of the Mpox vaccine acceptance rate was 61.1% (95% CI: 44.2-75.7%), with high heterogeneity (I² = 99%). Additionally, a pooled vaccine hesitancy prevalence was 13.2%, (95% CI: 2.4-48.6%), reflecting substantial variability and had high heterogeneity (I² = 98%).
Conclusion: This systematic review and meta-analysis reveal moderate Mpox vaccine acceptance and considerable hesitancy among PLWH. To further increase vaccine uptake and address any remaining hesitancy in this at-risk population, targeted public health strategies and ongoing research are necessary. Strengthening vaccine acceptance is critical to safeguarding PLWH against emerging infectious diseases such as Mpox.
Clinical trial number: Not applicable.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable, as there were no human participants involved in this study. Human Ethics and Consent to Participate Declarations: Not applicable. This study did not involve human subjects, so Human Ethics and Consent to Participate declarations are not required. Consent for Publication: Not applicable, as this study does not involve any individual person’s data in any form. Competing interests: The authors declare no competing interests.
Figures





References
-
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11(5):510–20. - PubMed
-
- Upadhyay S. Testing viral infections. Viral infections and antiviral therapies. Elsevier; 2023. pp. 99–120.
-
- Zammitt N, Sandilands E. Essentials of Kumar and Clark’s clinical medicine E-Book: essentials of Kumar and Clark’s clinical medicine. E-Book: Elsevier Health Sciences; 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

