Endogenous TDP-43 mislocalization in a novel knock-in mouse model reveals DNA repair impairment, inflammation, and neuronal senescence
- PMID: 40057796
- PMCID: PMC11889789
- DOI: 10.1186/s40478-025-01962-9
Endogenous TDP-43 mislocalization in a novel knock-in mouse model reveals DNA repair impairment, inflammation, and neuronal senescence
Abstract
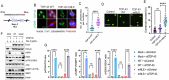
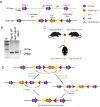
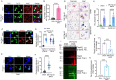
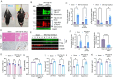
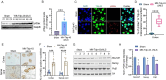
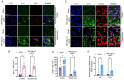
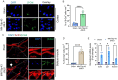
TDP-43 mislocalization and aggregation are key pathological features of amyotrophic lateral sclerosis (ALS)- and frontotemporal dementia (FTD). However, existing transgenic hTDP-43 WT or ∆NLS-overexpression animal models primarily focus on late-stage TDP-43 proteinopathy. To complement these models and to study the early-stage motor neuron-specific pathology during pre-symptomatic phases of disease progression, we generated a new endogenous knock-in (KI) mouse model using a combination of CRISPR/Cas9 and FLEX Cre-switch strategy for the conditional expression of a mislocalized Tdp-43∆NLS variant of mouse Tdp-43. This variant is expressed either in the whole body (WB) or specifically in the motor neurons (MNs) in two distinct models. These mice exhibit loss of nuclear Tdp-43, with concomitant cytosolic accumulation and aggregation in targeted cells, leading to increased DNA double-strand breaks (DSBs), signs of inflammation, and associated cellular senescence. Notably, unlike WT Tdp-43, which functionally interacts with Xrcc4 and DNA Ligase 4, the key DSB repair proteins in the non-homologous end-joining (NHEJ) pathway, the Tdp-43∆NLS mutant sequesters them into cytosolic aggregates, exacerbating neuronal damage in mouse brain. The mutant mice also exhibit myogenic degeneration in hindlimb soleus muscles and distinct motor deficits, consistent with the characteristics of motor neuron disease (MND). Our findings reveal progressive degenerative mechanisms in motor neurons expressing endogenous Tdp-43∆NLS mutant, independent of Tdp-43 overexpression or other confounding factors. Thus, this unique Tdp-43 KI mouse model, which displays key molecular and phenotypic features of Tdp-43 proteinopathy, offers a significant opportunity to characterize the early-stage progression of MND further and also opens avenues for developing DNA repair-targeted approaches for treating TDP-43 pathology-linked neurodegenerative diseases.
Keywords: Amyotrophic lateral sclerosis; DNA damage; Inflammation; Motor deficits; Motor neuron; Muscle atrophy; Neurodegeneration; Senescence; TDP-43.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Update of
-
Endogenous TDP-43 mislocalization in a novel knock-in mouse model reveals DNA repair impairment, inflammation, and neuronal senescence.Res Sq [Preprint]. 2024 Mar 20:rs.3.rs-3879966. doi: 10.21203/rs.3.rs-3879966/v2. Res Sq. 2024. Update in: Acta Neuropathol Commun. 2025 Mar 08;13(1):54. doi: 10.1186/s40478-025-01962-9. PMID: 38343852 Free PMC article. Updated. Preprint.
References
-
- Mackenzie IR, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9:995–1007. 10.1016/s1474-4422(10)70195-2 - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. 10.1126/science.1134108 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

