IL-1β stimulates ADAMTS9 expression and contributes to preterm prelabor rupture of membranes
- PMID: 40057799
- PMCID: PMC11890524
- DOI: 10.1186/s12964-025-02120-3
IL-1β stimulates ADAMTS9 expression and contributes to preterm prelabor rupture of membranes
Abstract
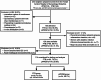
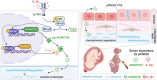
Background: Preterm prelabor rupture of membranes (pPROM) is a leading cause of neonatal morbidity and mortality. While intra-amniotic infection is a well-established driver of pPROM, the role of sterile intra-amniotic inflammation remains unclear. Recent evidence suggests that interleukin-1 beta (IL-1β) promotes extracellular matrix (ECM) remodeling via downstream effectors, a disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 9 (ADAMTS9), while protein O-fucosyltransferase 2 (POFUT2) facilitates its O-fucosylation and secretion, amplifying ECM degradation. This study investigates how IL-1β-triggered nuclear factor kappa-B (NF-κB) activation promotes ADAMTS9 and POFUT2 expression, ultimately driving fetal membrane ECM remodeling and weakening in pPROM without signs of intra-amniotic infection.
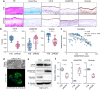
Methods: A nested case-control study included maternal serum and fetal membrane samples from 60 pregnant women (34 pPROM, 26 full-term births [FTB]). ELISA measured serum levels of IL-1β and ADAMTS9, and their correlations were analyzed. Mechanistic studies utilized primary human amniotic epithelial cells (hAECs) and fetal membrane-decidua explants with IL-1β treatment. The role of NF-κB was explored using chromatin immunoprecipitation (ChIP) and luciferase assays to assess NF-κB binding to the promoters of ADAMTS9 and POFUT2. A murine model of sterile intra-amniotic inflammation under ultrasound-guided IL-1β injection was used to validate in vitro findings and assess pregnancy outcomes.
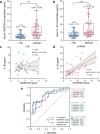
Results: Serum IL-1β and ADAMTS9 levels at 16 weeks of gestation were significantly higher in pPROM cases compared to FTB controls (P < 0.001). A combined model of these biomarkers demonstrated high predictive accuracy for pPROM (AUC = 0.83). Mechanistically, IL-1β activated NF-κB, leading to its binding to the promoters of ADAMTS9 and POFUT2. NF-κB activation promoted ADAMTS9 expression, while POFUT2 enhanced its secretion. Together, these processes drove versican degradation and ECM weakening. Intra-amniotic administration of IL-1β in mice induced fetal membrane weakening, preterm birth, and adverse neonatal outcomes, which were mitigated by the NF-κB inhibitor BAY 11-7082 treatment.
Conclusion: Maternal serum ADAMTS9 levels at mid-gestation are promising non-invasive biomarkers for pPROM risk stratification. Mechanistically, IL-1β-induced NF-κB activation promotes ADAMTS9 expression and POFUT2-dependent secretion, contributing to fetal membrane weakening. These findings provide new insights into the role and potential therapeutic target for sterile intra-amniotic inflammation in pPROM.
Keywords: ADAMTS9; Interleukin-1 beta; NF-kappa B; Preterm prelabor rupture of fetal membranes; Protein O-fucosyltransferase 2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Upon the declaration of Helsinki, the clinical sample was approved by the Obstetrics and Gynecology Ethics Committee of Tianjin Central Hospital (Approval No. 2021KY113). The confidentiality of the patients was strictly protected, and no personal data were required for this study. All participants provided written informed consent. All animal procedures, including euthanasia, were carried out following ARRIVE guidelines and approved by the Institutional Review Board of Nankai University. Consent for publication: All authors have agreed to publish this manuscript. Competing interests: The authors declare no competing interests.
Figures





References
-
- Liu Y, Gao L. Preterm labor, a syndrome attributed to the combination of external and internal factors. Maternal-Fetal Med. 2022;4(1):61–71. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

