Comparative Effectiveness of Different Opioid Regimens, in Daily Dose or Treatment Duration, Prescribed at Surgical Discharge: a Systematic Review and Meta-Analysis
- PMID: 40057907
- PMCID: PMC11909025
- DOI: 10.1007/s40263-025-01165-9
Comparative Effectiveness of Different Opioid Regimens, in Daily Dose or Treatment Duration, Prescribed at Surgical Discharge: a Systematic Review and Meta-Analysis
Abstract
Background: Opioids are prescribed for postsurgical pain management, but a balance between achieving adequate pain control and minimising opioid-related harm is required. This study aimed to investigate the effectiveness of different opioid regimens, in daily dose or treatment duration, prescribed at surgical discharge.
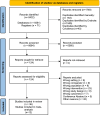
Methods: A systematic search of MEDLINE, EMBASE, CENTRAL, and ICTRP was performed from inception to 12 January 2025. Randomised controlled trials (RCTs) and non-RCTs comparing different daily doses or treatment durations of opioid analgesics were included. All surgeries were included, except those related to cancer treatment or palliative care. Eligible populations were adults (≥ 18 years) or individuals classified as adults according to the criteria of the respective studies. Data were extracted at immediate-term (≤ 3 days), short-term (> 3 to ≤ 7 days), medium-term (> 7 to ≤ 30 days), and long-term (> 30 days). Data from RCTs were pooled using a random-effects model. Risk of bias was assessed. Certainty of evidence from RCTs was evaluated with Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). The primary outcome was pain intensity. Adverse events were also measured.
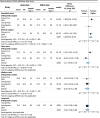
Results: A total of 8432 records were identified. In total, 12 RCTs with 7128 patients and 24 non-RCTs with 118,849 patients were included. Studies included orthopaedic, gynaecology and obstetric surgeries, ranging from minor to major procedures. Higher-doses of opioids were more effective than lower-doses in reducing immediate pain intensity (mean difference (MD) 4.36, 95% confidence interval (CI) 0.50-8.23, n = 364, three studies, I2 = 0%, high certainty). No difference in pain was found between higher-doses and lower-doses at other time points (moderate to high certainty). Longer-durations of opioid treatment showed no difference in pain at any time point (low to moderate certainty). More adverse events were reported with higher doses of opioids.
Conclusions: Higher-dose opioids provide a slight reduction in immediate post-discharge pain intensity but may lead to more adverse events. Longer durations of opioid treatment are probably not more effective in reducing pain than shorter treatment durations. Our findings suggest that clinicians may choose to prescribe lower doses of opioids or shorter durations of opioids without compromising pain control, even for major surgery.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Masoud Jamshidi, Caitlin MP Jones, Aili V Langford, Asad E Patanwala, Chang Liu, Ian A Harris, Janney Wale, Mark Horsley, Sam Adie, Deanne Jenkin and Chung-Wei Christine Lin have no conflicts of interest that are directly relevant to the content of this article. Availability of data and material: All data generated or analysed during this study are included in this published article (and its supplementary information files). Ethical approval: Not applicable. Funding: C-WCL and AVL are funded by fellowships from the National Health and Medical Research Council, Australia. Open access fee was supported by the University of Sydney. Consent to participate: Not applicable. Consent for publication: Not applicable Code availability: Not applicable. Author contributions: C.-W. C.L. was involved in conceptualisation, supervision, interpretation and critical revision of the manuscript; M.J. was involved in conceptualisation, searching, screening, extraction, analysis and writing the first draft of manuscript; C.M.P.J. and A.V.L. were involved in interpretation, searching, screening, extraction, analysis and critical revision of the manuscript; A.E.P. was involved in conceptualisation and critical revision of the manuscript; C.L., I.A.H., M.H. and S.A. were involved in critical revision of the manuscript; J.W. was involved as a consumer representative and in the critical revision of the manuscript; D.E.J. was involved in updating the screening, extraction, analysis and critical revision of the manuscript. All authors have approved the final version of the manuscript.
Figures


References
-
- Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, et al. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet. 2016;387(10028):1644–56. 10.1016/s0140-6736(16)00161-6. - PubMed
-
- World Health Organization. Opioid overdose. 2021 [cited 2022 Dec 14]. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose.
-
- Campbell G, Nielsen S, Larance B, Bruno R, Mattick R, Hall W, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. 2015;16(9):1745–58. 10.1111/pme.12773. - PubMed
-
- Humphreys K. Avoiding globalisation of the prescription opioid epidemic. Lancet (London, England). 2017;390(10093):437–9. 10.1016/s0140-6736(17)31918-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

