Spatial characterization of RPE structure and lipids in the PEX1-p.Gly844Asp mouse model for Zellweger spectrum disorder
- PMID: 40058592
- PMCID: PMC11999432
- DOI: 10.1016/j.jlr.2025.100771
Spatial characterization of RPE structure and lipids in the PEX1-p.Gly844Asp mouse model for Zellweger spectrum disorder
Abstract
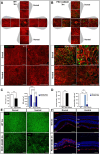
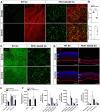
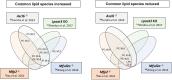
Zellweger Spectrum Disorder (ZSD) is caused by defects in PEX genes, whose proteins are required for peroxisome assembly and function. Peroxisome dysfunction in ZSD causes multisystem effects, with progressive retinal degeneration (RD) among the most frequent clinical findings. However, much remains unknown about how peroxisome deficiency causes RD. To study RD pathophysiology in ZSD, we used the PEX1-p.Gly844Asp (G844D) mouse model, which represents the common human PEX1-p.Gly843Asp (G843D) variant. We previously reported diminished retinal function, diminished functional vision, and neural retina structural defects in this model. Here, we investigate the retinal pigment epithelium (RPE) phenotype, examining morphological, inflammatory, and lipid changes at 1, 3, and 6 months of age. We report that RPE cells exhibit evident degeneration by 3 months that worsens with time, starts in the dorsal pole, and is accompanied by subretinal inflammatory cell infiltration. We match these events with imaging mass spectrometry for regional analysis of lipids in the RPE. We identified 47 lipid alterations preceding structural changes, 9 of which localize to the dorsal pole. 29 of these persist to 3 months, with remodeling of the dorsal pole lipid signature. 13 new alterations occur concurrent with histological changes. Abnormalities in peroxisome-dependent lipids detected by LC/MS/MS are exacerbated over time. This study represents the first characterization of RPE in a ZSD model, and the first in situ lipid analysis in peroxisome-deficient tissue. Our findings uncover potential lipid drivers of RD progression in ZSD, and identify candidate biomarkers for retinopathy progression and response to therapy.
Keywords: PEX1; dyslipidemia; eye/retina; inflammation; lipidomics; lipids; mass spectrometry imaging; peroxisome disease; retinal degeneration; retinal pigment epithelium.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



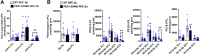
References
-
- Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 2012;1822:1442–1452. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

