Predictors of Long-Term Survival in Patients with Idiopathic Pulmonary Fibrosis: Data from the IPF-PRO Registry
- PMID: 40059108
- PMCID: PMC11891094
- DOI: 10.1007/s00408-025-00797-4
Predictors of Long-Term Survival in Patients with Idiopathic Pulmonary Fibrosis: Data from the IPF-PRO Registry
Abstract
Purpose: We used data from the IPF-PRO Registry of patients with idiopathic pulmonary fibrosis (IPF) to identify characteristics that predicted survival for a further > 5 years.
Methods: Participants had IPF that was diagnosed or confirmed at the enrolling center in the previous 6 months. Patients were followed prospectively. A Classification And Regression Tree (CART) was used to identify predictors of survival > 5 versus ≤ 5 years following enrollment. The following variables, assessed at enrollment, were considered: age; body mass index (BMI); former smoker; current smoker; time from first imaging evidence, symptoms, or diagnosis of IPF to enrollment; forced vital capacity (FVC) % predicted; diffusing capacity of the lungs for carbon monoxide (DLco) % predicted; antifibrotic drug use; supplemental oxygen use; history of cardiac disease; pulmonary hypertension; COPD/emphysema; and rural location.
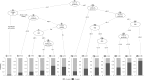
Results: The analysis cohort comprised 819 patients, of whom 278 (33.9%) survived > 5 years. DLco % predicted, supplemental oxygen use and FVC % predicted were the most important variables for predicting survival > 5 versus ≤ 5 years after enrollment. The importance of these variables (scaled such that the most important had an importance of 100%) was 100%, 78.2% and 74.2%, respectively. The optimism-corrected area under the curve (AUC) of the CART was 0.72, with an accuracy of 0.72.
Conclusion: Among patients enrolled in the IPF-PRO Registry, a decision tree that included DLco % predicted, oxygen use and FVC % predicted facilitated the prediction of survival > 5 years. Understanding predictors of longer-term survival may facilitate conversations with patients about their prognosis and treatment.
Keywords: Interstitial lung diseases; Mortality; Observational study; Prognosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: HJK is a principal investigator at a site in the IPF-PRO/ILD-PRO Registry. HJK has received research support from Boehringer Ingelheim, FibroGen, Galapagos, the National Institutes of Health, Roche/Genentech, United Therapeutics. JMW, MLN and LDS are employees of the Duke Clinical Research Institute, which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc to run the IPF-PRO/ILD-PRO Registry. JLT also reports grants from AstraZeneca and CareDx and participation in advisory boards for Altavant, Avalyn, Natera, Sanofi, Theravance. AHC is a principal investigator at a site in the IPF-PRO/ILD-PRO Registry. AHC has received research support from Boehringer Ingelheim, Roche/Genentech, United Therapeutics, FibroGen, Veracyte, Galapagos, Pilant, Kadmon, Bristol Myers Squibb, Bellerophon, Pulmonary Fibrosis Foundation (PFF), Galecto; consulting fees from Veracyte; speaker fees from Boehringer Ingelheim, Genentech, The France Foundation, Paradigm Medical; medical writing support from Boehringer Ingelheim, Veracyte, United Therapeutics; and has acted as a medical advisor to the PFF. AHJ has no disclosures. PL and ALO are employees of Boehringer Ingelheim Pharmaceuticals, Inc. Ethical Approval: The study was approved by the Duke University Institutional Review Board (Pro00046131). The protocol was approved by the relevant Institutional Review Boards and/or local Independent Ethics Committees prior to patient enrollment at every site (listed in the Acknowledgements). Consent to Participate: All patients provided written consent prior to entering the registry. Consent for Publication: Not applicable.
Figures

References
-
- Fainberg HP, Oldham JM, Molyneaux PL et al (2022) Forced vital capacity trajectories in patients with idiopathic pulmonary fibrosis: a secondary analysis of a multicentre, prospective, observational cohort. Lancet Digit Health 4:e862–e872 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

