Automated segmentation of the sacro-iliac joints, posterior spinal joints and discovertebral units on low-dose computed tomography for Na[18F]F PET lesion detection in spondyloarthritis patients
- PMID: 40059265
- PMCID: PMC11891110
- DOI: 10.1186/s40658-025-00734-7
Automated segmentation of the sacro-iliac joints, posterior spinal joints and discovertebral units on low-dose computed tomography for Na[18F]F PET lesion detection in spondyloarthritis patients
Abstract
Purpose: Spondyloarthritis (SpA) is a chronic inflammatory rheumatic disease which involves the axial skeleton. Quantitative sodium fluoride-18 (Na[18F]F) PET/CT is a new imaging approach promising for accurate diagnosis and treatment monitoring by assessment of molecular bone pathology in SpA. Detection of Na[18F]F PET positive lesions is time-consuming and subjective, and can be replaced by automatic methods. This study aims to develop and validate an algorithm for automated segmentation of the posterior spinal joints, sacro-iliac joints (SIJs) and discovertebral units (DVUs) on low-dose computed tomography (LDCT), and to employ these segmentations for threshold-based lesion detection.
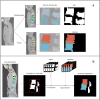
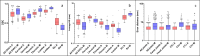
Methods: Two segmentation methods were developed using Na[18F]F PET/LDCT images from SpA patients. The first method employed morphological operations to delineate the joints and DVUs, while the second used a multi-atlas-based approach. The performance and reproducibility of these methods were assessed on ten manually segmented LDCTs using average Hausdorff distance (HD) and dice similarity coefficient (DSC) for DVUs and SIJs, and mean error distance for the posterior joints. Various quantitative PET metrics and background corrections were compared to determine optimal lesion detection performance relative to visual assessment.
Results: The morphological method achieved significantly better DSC (0.82 (0.73-0.88) vs. 0.74 (0.68-0.79); p < 0.001) for all DVUs combined compared to the atlas-based method. The atlas-based method outperformed the morphological method for the posterior joints with a median error distance of 4.00 mm (4.00-5.66) vs. 5.66 mm (4.00-8.00) (p < 0.001). For lesion detection, the atlas-based segmentations were more successful than the morphological method, with the most accurate metric being the maximum standardized uptake value (SUVmax) of the lesional Na[18F]F uptake, corrected for the median SUV (SUVmedian) of the spine, with an area under the curve of 0.90.
Conclusion: We present the first methods for detailed automatic segmentation of the posterior spinal joints, DVUs and SIJs on LDCT. The atlas-based method is the most appropriate, reaching high segmentation performance and lesion detection accuracy. More research on the PET-based lesion segmentation is required, to develop a pipeline for fully automated lesional Na[18F]F uptake quantification.
Keywords: Artificial intelligence-based segmentation; Automated lesion detection; PET quantification; Rheumatic disease; Spinal bone formation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval was granted by the Ethics Committee of the Amsterdam University Medical Center (2013/38). All patients gave written informed consent to participate in the study, according to the regulations of the Medical Ethical Committee of Amsterdam University Medical Center. Consent for publication: Not applicable. Competing interests: WvdH, FvV, RH,TD, RB, CvdL, GZ: no competing interests to declare.
Figures




Similar articles
-
Automated multiclass segmentation, quantification, and visualization of the diseased aorta on hybrid PET/CT-SEQUOIA.Med Phys. 2024 Jun;51(6):4297-4310. doi: 10.1002/mp.16967. Epub 2024 Feb 7. Med Phys. 2024. PMID: 38323867
-
Automatic segmentation of prostate cancer metastases in PSMA PET/CT images using deep neural networks with weighted batch-wise dice loss.Comput Biol Med. 2023 May;158:106882. doi: 10.1016/j.compbiomed.2023.106882. Epub 2023 Apr 4. Comput Biol Med. 2023. PMID: 37037147
-
A computational pipeline for quantification of pulmonary infections in small animal models using serial PET-CT imaging.EJNMMI Res. 2013 Jul 23;3(1):55. doi: 10.1186/2191-219X-3-55. EJNMMI Res. 2013. PMID: 23879987 Free PMC article.
-
Semi-automated segmentation methods of SSTR PET for dosimetry prediction in refractory meningioma patients treated by SSTR-targeted peptide receptor radionuclide therapy.Eur Radiol. 2023 Oct;33(10):7089-7098. doi: 10.1007/s00330-023-09697-8. Epub 2023 May 6. Eur Radiol. 2023. PMID: 37148355 Review.
-
Performance Evaluation of a Semi-automated Method for [18F]FDG Uptake in Abdominal Visceral Adipose Tissue.Mol Imaging Biol. 2019 Feb;21(1):159-167. doi: 10.1007/s11307-018-1211-1. Mol Imaging Biol. 2019. PMID: 29789994
Cited by
-
Current application, possibilities, and challenges of artificial intelligence in the management of rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis.Ther Adv Musculoskelet Dis. 2025 Jun 21;17:1759720X251343579. doi: 10.1177/1759720X251343579. eCollection 2025. Ther Adv Musculoskelet Dis. 2025. PMID: 40547599 Free PMC article. Review.
References
-
- Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377(9783):2127–37. - PubMed
-
- Mease PJ. Suspecting and diagnosing the patient with spondyloarthritis and what to expect from therapy. Rheum Dis Clin North Am. 2022;48(2):507–21. - PubMed
-
- Zhao SS, Pittam B, Harrison NL, Ahmed AE, Goodson NJ, Hughes DM. Diagnostic delay in axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(4):1620–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

