The impact of electronic cigarettes on airway epithelial barrier integrity in preclinical mouse model
- PMID: 40059636
- PMCID: PMC12147400
- DOI: 10.1152/ajplung.00408.2024
The impact of electronic cigarettes on airway epithelial barrier integrity in preclinical mouse model
Abstract
The increasing use of electronic cigarettes (e-cigs) among adolescents poses significant public health risks. This study investigates the impact of e-cigs on the airway epithelial barrier, focusing on apical junctional complexes (AJCs), including tight junctions (TJs) and adherens junctions (AJs). We hypothesized that e-cigs disrupt AJCs in a mouse model, leading to increased airway barrier permeability. C57BL/6 mice were exposed to 36 mg/mL e-cig aerosols (3 puffs/min) for 1 h daily over 4 days. Bronchoalveolar lavage (BAL) fluid analysis, lung inflammation assessment, immunohistochemistry (IHC) staining, Western blotting (WB), and permeability assays were performed to evaluate the structure and function of the airway barrier. E-cig-exposed mice showed weight loss and elevated serum cotinine levels. BAL fluid analysis revealed elevated white blood cells. Histological analysis confirmed lung inflammation, whereas IHC and WB showed significant AJC disruption. Notably, claudin-2 levels were elevated in e-cig-exposed mice compared with controls. Claudin-2, known for its role in promoting permeability in "leaky" epithelia, increased alongside decreases in other TJ components, signifying structural barrier impairment. After e-cig exposure, instilling fluorescein isothiocyanate (FITC)-dextran into the airway increased serum FITC-dextran levels, indicating enhanced barrier permeability. E-cig aerosol exposure disrupts airway epithelial barrier structure and function, primarily through the disassembly of TJs and AJs. These findings suggest potential pathways for further clinical investigation into the health risks of e-cig use.NEW & NOTEWORTHY The rising use of e-cigs among youth has become a significant public health concern. This study, using a mouse model, demonstrates that exposure to e-cig aerosol leads to airway inflammation, structural damage to the airway epithelial barrier, and increased epithelial barrier permeability.
Keywords: FITC—fluorescein isothiocyanate; airway epithelial barrier; electronic cigarette; immunohistochemistry; vaping.
Conflict of interest statement
DISCLOSURES
The authors report no conflicts of interest.
Figures
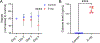



References
-
- Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH , and Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314: R834–R847, 2018. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

