CpG oligodeoxynucleotides and pan-serotype inhibitors control neurotropic dengue infection in novel immune competent neonatal mouse model
- PMID: 40059770
- PMCID: PMC12269057
- DOI: 10.1080/22221751.2025.2477668
CpG oligodeoxynucleotides and pan-serotype inhibitors control neurotropic dengue infection in novel immune competent neonatal mouse model
Abstract
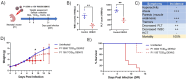
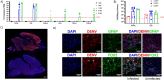
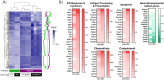
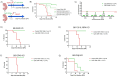
Dengue virus (DENV) is a growing global public health threat. The lack of symptomatic immune-competent animal models for dengue has hindered the screening and development of effective therapeutics that can be used to control dengue virus replication and thereby control the progression to severe dengue disease. To address this, we established an infection model in neonatal C57BL/6 mice and showed that a systemic Dengue challenge leads to ataxia, seizures, paralysis, and death within 15 days. The virus was found predominantly in the eye and brain where DENV infects neurons but not astrocytes and causes extensive infiltration of macrophages and microglia activation. The response to infection included upregulation of multiple genes linked to interferons (Ifna, Ifnb, Ifng, Irf7, Irf8, Mx1, Stat1 and Bst2), inflammation (Il6,Tnfa), complement (Cfb,C1ra,C2, C3), cytolysis (Gzma, Gzmb, Prf1) consistent with antiviral responses and inflammation together with neuroprotective regulatory signals (Il27, Il10, and stat2). The increased proinflammatory signature was associated with downregulation neurodevelopmental genes (Calb2, Pvalb, Olig1 and Olig2). We tested the utility of this mouse model by assessing the protection conferred by direct antivirals JNJ-A07 and ST-148 and host-directed antiviral immunomodulatory CpG oligodeoxynucleotide (ODN), alone or in combination against lethal dengue viral infection. The data showed that immunomodulatory CpG ODN and antiviral JNJ-A07 improved the survival of neonatal mice, and protection from lethal neurotropic infection was optimal when treatments were combined. This study suggests that a combination of an effective dengue antiviral along with a host-directed therapeutic may be a useful strategy to protect against dengue virus infections.
Keywords: Dengue; immunocompetent; immunomodulatory; mouse model; neurotropic; virus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Vitamin D enhances antiviral responses in dengue virus-infected macrophages by modulating early-response gene expression.PLoS One. 2025 Aug 21;20(8):e0330751. doi: 10.1371/journal.pone.0330751. eCollection 2025. PLoS One. 2025. PMID: 40839599 Free PMC article.
-
Sequential macrophage DENV and ZIKV infection shows differential expression of CD86, IFN-β, and regulation of TNF-α and IL-1β depending on DENV serotype.Braz J Microbiol. 2025 Jun;56(2):1083-1094. doi: 10.1007/s42770-025-01639-4. Epub 2025 Feb 19. Braz J Microbiol. 2025. PMID: 39969815
-
Pharmacological inhibition of the RhoA pathway by melatonin reduces viral replication and proinflammatory response against ZIKV and DENV-4 neuroinfections.Front Immunol. 2025 Aug 1;16:1630116. doi: 10.3389/fimmu.2025.1630116. eCollection 2025. Front Immunol. 2025. PMID: 40821819 Free PMC article.
-
Antiviral agents for infectious mononucleosis (glandular fever).Cochrane Database Syst Rev. 2016 Dec 8;12(12):CD011487. doi: 10.1002/14651858.CD011487.pub2. Cochrane Database Syst Rev. 2016. PMID: 27933614 Free PMC article.
-
Antiviral treatment for Bell's palsy (idiopathic facial paralysis).Cochrane Database Syst Rev. 2015 Nov 9;(11):CD001869. doi: 10.1002/14651858.CD001869.pub8. Cochrane Database Syst Rev. 2015. PMID: 26559436
References
-
- WHO . Dengue and severe dengue 2024 [cited 2024 Jul 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
