Evaluation of prognostic scores in patients with HCC undergoing first-line immunotherapy with atezolizumab and bevacizumab
- PMID: 40059970
- PMCID: PMC11889551
- DOI: 10.1016/j.jhepr.2024.101295
Evaluation of prognostic scores in patients with HCC undergoing first-line immunotherapy with atezolizumab and bevacizumab
Abstract
Background & aims: Immunotherapy with atezolizumab and bevacizumab (a + b) has improved the prognosis of patients with unresectable hepatocellular carcinoma (HCC). However, the outcome for individual patients is highly variable. This study aimed to (i) develop and validate a prognostic prediction model to estimate individual prognosis and (ii) compare it with established models.
Methods: In this multicenter retrospective study, patients with HCC undergoing first-line immunotherapy with a + b from 24 centers (Europe, USA) were included. Statistical analysis and reporting followed the TRIPOD guidelines. The primary objective was overall survival (OS). A Cox model was developed and externally validated.
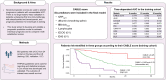
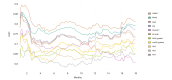
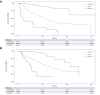
Results: In total, 683 patients were included (training: 526, validation: 157). The C-reactive protein, albumin, bilirubin, lymphocytes, ECOG performance status, and extrahepatic spread (CABLE score) remained significantly associated with OS in Cox regression analysis. In the training set, the CABLE score had a higher discriminatory accuracy relative to ALBI, EZ-ALBI, mALBI, CRAFITY, PNI, NLR, PLR, and GPS (time-dependent AUC 0.79 and C-index 0.75 (95% CI 0.71-0.78) at 12 months). In the external validation set, the discriminatory performance of the CABLE score was comparable to ALBI, EZ-ALBI, and mALBI, but on average higher than PNI, CRAFITY, NLR, PLR, and GPS. In patients with Child-Pugh A, the CABLE score outperformed ALBI, EZ-ALBI, and mALBI in the first 9 months. We provide a web-based calculator for the CABLE score to allow estimation of individual prognosis for these patients (http://shiny.imbei.uni-mainz.de:3838/CABLE_Score/).
Conclusions: The CABLE score shows good discriminatory performance in assessing the individual prognosis of patients undergoing first-line immunotherapy with a + b. Further validation studies are needed to investigate its performance compared with the ALBI score, in particular in subgroup analysis.
Impact and implications: The CABLE score allows estimation of the prognosis of patients with unresectable hepatocellular carcinoma undergoing first-line immunotherapy with atezolizumab and bevacizumab at an individual level using our web-based calculator. This feature, as well as the evaluation of the score's added benefit through an extensive comparison with other established scores, can inform clinicians on their significance and may guide clinical decision-making in the context of a malignant disease where the prognosis has become highly variable. Further large validation studies are needed to investigate the incremental value of the CABLE score compared with the ALBI score, in particular in subgroups such as patients designated as Child-Pugh A.
Keywords: CABLE score; Cirrhosis; Development; Hepatocellular carcinoma; Immune checkpoint inhibitors; Immunotherapy; Outcome; PD-L1; Prediction model; Systemic therapy; VEGF; Validation.
© 2024 The Author(s).
Conflict of interest statement
SJG has received travel expenses from Ipsen and Gilead. NA has received travel support and speaker fees from AbbVie and Gilead. FFo has received honoraria for lectures from AstraZeneca, Lilly, MSD, Pfizer, and Roche. He has served as advisory board member to AstraZeneca, BMS, Eisai, and Roche and has received travel support from Merck KGaA and Servier. JUM received honoraria from AstraZeneca, Eisai, MSD, MERZ, AbbVie, and Roche. DB has received lecture and speaker fees from W.L. Gore & Associates GmbH, the Falk Foundation Germany, and a travel grant from Gilead Science. KB has received honoraria for lectures from Ipsen. NHT has received honorarium (to the institution) from Helsinn and is a consultant for AZ, Genentech, and TEMPUS. FPR has received honoraria for lectures, consulting activities, and travel support from the Falk Foundation, AbbVie, Gilead, Ipsen, AstraZeneca, Roche, and Novartis. EDT has served as a paid consultant for AstraZeneca, Bayer, BMS, Eisai, Eli Lilly & Co, Pfizer, Ipsen, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, AstraZeneca, BMS, Bayer, Celsion, and Roche, and lecture honoraria from BMS and Falk. In addition, he has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. NBK has received reimbursement of meeting attendance fees and travel expenses from Eisai and a lecture honorarium from Falk and AstraZeneca. KS has received honoraria for lectures from AstraZeneca, MSD. He has served as advisory board member to AstraZeneca, MSD, Servier, Eisai, and Roche. LM has received an honorarium for lecture from Bayer. UE has received honoraria for lectures from AstraZeneca, Eisai, the Falk Foundation, Ipsen, MSD, and Novartis. She has served as advisory board member to AstraZeneca, Bayer, MSD, and Eisai and has received travel support from AstraZeneca and Biotest. LR received consulting fees from AstraZeneca, Basilea, Bayer, BMS, Eisai, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, Zymeworks; lecture fees from AstraZeneca, Bayer, BMS, Eisai, Incyte, Ipsen, Merck Serono, Roche, Servier; travel expenses from AstraZeneca; research grants (to Institution) from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Zymeworks. AD received educational support for congress attendance and consultancy fees from Roche, and speaker fees from Roche, AstraZeneca, Eisai, and Chugai. FFi has received travel support from Ipsen, AbbVie, AstraZeneca and speaker’s fees from AbbVie, MSD, Ipsen, Astra. MP received speaker honoraria from Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he is a consultant for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he received grants from Roche and BMS; he received travel support from Bayer, BMS, Ipsen, and Roche. MPR has received consulting fees from Bayer, BMS, Boehringer-Ingelheim, Eisai, Gilead, Intercept-Advanz, Ipsen, Lilly, MSD, Roche, Sanofi and was an investigator for Bayer, BMS, Boehringer-Ingelheim, Exelixis, Eisai, Falk, Gilead, Lilly, Ipsen, Novartis, and Roche. DJP received lecture fees from Bayer Healthcare, Eisai, BMS, Roche, Boston Scientific, travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, DaVolterra, Mursla, Ipsen, Exact Sciences, Avamune, Eisai, Roche, Starpharma, LiFT biosciences and AstraZeneca; received research funding (to institution) from MSD, GSK, and BMS. LSJ received speaker honoraria from Falk Foundation, Boston Scientific, AbbVie, and AstraZeneca. She is consultant for Boston Scientific and AstraZeneca. She received travel support from Biotest and AbbVie. MAGC has contributed to advisory boards for Roche, Eisai, MSD, BMS, AZ, Amgen and Servier (these activities have no potential conflicts of interest with the manuscript). AW received compensations as a member of scientific advisory boards for Bayer, BMS, Eisai, and Sanofi and served as a speaker for Leo Pharma, Eisai, Ipsen, and Roche and received travel support from Merck and Servier. RK has received consultancy fees from Boston Scientific, Bristol-Myers Squibb, Guerbet, Roche, and SIRTEX, and lecture fees from AstraZeneca, BTG, Eisai, Guerbet, Ipsen, Roche, Siemens, SIRTEX, and MSD Sharp & Dohme (none are related to this work). PRG received honoraria for lectures and advisory boards from Bayer, BMS, MSD, AstraZeneca, Lilly, Ipsen, Roche, Eisai, Guerbet, and Boston Scientific. SII served as advisory board member for AstraZeneca and received consulting fees from AstraZeneca. All other authors disclose no potential financial or non-financial conflict of interests. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Finn R.S., Qin S., Ikeda M., et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39(3 Suppl):267.
-
- Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. - PubMed
-
- Cheng A.-L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

