Antibody Response to Covishield and Covaxin in Kidney Transplant Recipients
- PMID: 40060063
- PMCID: PMC11883331
- DOI: 10.25259/ijn_549_23
Antibody Response to Covishield and Covaxin in Kidney Transplant Recipients
Abstract
Background: The COVID-19 pandemic had a major impact on solid organ transplant recipients. COVID-19 vaccination plays a crucial role in pandemic management.There is limited data on replication-defective viral vectors [ChAdOx1-nCOV (COVISHIELDTM)] and whole inactivated one BBV-152 (COVAXINTM) in kidney transplant recipients (KTRs). This study aims to assess the humoral immune response and adverse effects of these vaccines in KTRs after the first and second doses of vaccination.
Materials and methods: Anti-SARS-CoV-2 anti-spike antibody titers were measured in 285 KTRs recipients prior to vaccination, 3 weeks ± 3 days after first dose and 3 weeks ± 3 days after second dose of the COVISHIELD (n = 232) and COVAXIN (n = 55) vaccines. Anti-spike antibodies were measured by the chemiluminescence immunoassay method. The primary outcome was seroconversion after two doses of COVAXIN and COVISHIELD and secondary outcome was the incidence of adverse events to COVID-19 vaccines within one week of vaccination.
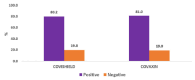
Results: At baseline, 25 (39.7%) and 67 (30.2%) of KTRs were found to be seropositive before receiving COVAXINTM and COVISHIELDTM, respectively. After first dose of vaccination, 46 (73.0%) and 158 (71.2%) were seropositive and after second dose, 51 (81.0%) and 177 (79.7%) were seropositive, respectively. Common adverse effects were fever, chills, myalgia, and headache which settled in 1-2 days. There was no episode of rejection.
Conclusion: Both ChAdOx1-nCOV and BBV-152 were well tolerated and induced robust antibody formation in KTRs in the Indian population.
Keywords: COVAXIN; COVID vaccine; COVISHIELD; Humoral response; India; Kidney transplant; Seropositivity.
© 2025 Indian Journal of Nephrology | Published by Scientific Scholar.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- World Health Organization WHO coronavirus disease (COVID-19) dashboard. Available from https://covid19.who.int/ [Accessed on March 05, 2023]
-
- Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492–509. doi: 10.1016/j.vaccine.2021.09.055. - DOI - PMC - PubMed
-
- Aziz F, Mandelbrot D, Singh T, Parajuli S, Garg N, Mohamed M, et al. Early report on published outcomes in kidney transplant recipients compared to nontransplant patients infected with coronavirus disease 2019. Transplant Proc. 2020;52:2659–62. doi: 10.1016/j.transproceed.2020.07.002. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous