Asymmetric copper-catalyzed hydrophosphinylation of ethynylazaarenes to access P-chiral 2-azaaryl-ethylphosphine oxides
- PMID: 40060098
- PMCID: PMC11886619
- DOI: 10.1039/d5sc00358j
Asymmetric copper-catalyzed hydrophosphinylation of ethynylazaarenes to access P-chiral 2-azaaryl-ethylphosphine oxides
Abstract
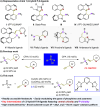
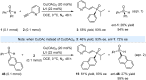
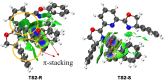
We report a cost-effective approach for the enantioselective hydrophosphinylation of ethynylazaarenes utilizing a chiral copper catalytic platform. This strategy efficiently converts racemic secondary phosphine oxides (SPOs) into P-chiral tertiary phosphine oxides (TPOs) bearing functionalized olefin substituents with azaarene moieties, achieving high yields and exceptional enantioselectivities. These adducts serve as crucial intermediates in the development of valuable chiral 1,5-hybrid P,N-ligands. The facile introduction of diverse additional carbon-centered chirality through the transformation of the olefin moiety effectively enhances the enantioselectivity of asymmetric metal catalysis compared to ligands exhibiting solely P-chirality. Mechanistic investigations reveal that the interaction between the chiral Cu(i) complex and azaarenes promotes the kinetic resolution of SPOs. The robustness of this method is further demonstrated by its ability to incorporate deuterium atoms into the olefins, highlighting its potential relevance in pharmaceutical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

