This is a preprint.
β-Amyloid Induces Microglial Expression of GPC4 and APOE Leading to Increased Neuronal Tau Pathology and Toxicity
- PMID: 40060520
- PMCID: PMC11888210
- DOI: 10.1101/2025.02.20.637701
β-Amyloid Induces Microglial Expression of GPC4 and APOE Leading to Increased Neuronal Tau Pathology and Toxicity
Update in
-
β-Amyloid induces microglial expression of GPC4 and APOE leading to increased neuronal tau pathology and toxicity.Mol Neurodegener. 2025 Aug 29;20(1):96. doi: 10.1186/s13024-025-00883-4. Mol Neurodegener. 2025. PMID: 40883746 Free PMC article.
Abstract
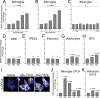
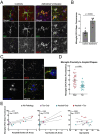
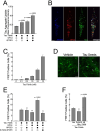
To elucidate the impact of Aβ pathology on microglia in Alzheimer's disease pathogenesis, we profiled the microglia surfaceome following treatment with Aβ fibrils. Our findings reveal that Aβ-associated human microglia upregulate Glypican 4 (GPC4), a GPI-anchored heparan sulfate proteoglycan (HSPG). In a Drosophila amyloidosis model, glial GPC4 expression exacerbates motor deficits and reduces lifespan, indicating that glial GPC4 contributes to a toxic cellular program during neurodegeneration. In cell culture, GPC4 enhances microglia phagocytosis of tau aggregates, and shed GPC4 can act in trans to facilitate tau aggregate uptake and seeding in neurons. Additionally, our data demonstrate that GPC4-mediated effects are amplified in the presence of APOE. These studies offer a mechanistic framework linking Aβ and tau pathology through microglial HSPGs and APOE.
Keywords: APOE; Alzheimer’s disease; Neurodegeneration; amyloid; astrocytes; dementia; drosophila; microglia; seeding; tau.
Conflict of interest statement
Competing interests B.B.H has the following US patents: Therapeutic Anti-Tau Antibodies (US9834596), Method for Detection of Aggregates in Biological Samples (US9910048) and Methods and Compositions Related to Heparinoids (WO2021142312A1). M.K. is a co-scientific founder of Montara Therapeutics and serves on the Scientific Advisory Boards of Engine Biosciences, Casma Therapeutics, Alector, and Montara Therapeutics, and is an advisor to Modulo Bio and Recursion Therapeutics.
Figures




References
-
- Musiek E. S. & Holtzman D. M. Origins of Alzheimer’s disease: reconciling cerebrospinal fluid biomarker and neuropathology data regarding the temporal sequence of amyloid-beta and tau involvement. Current Opinion in Neurology Publish Ahead of Print, 10.1097/WCO.0b013e32835a30f4 (2012). - DOI - PMC - PubMed
-
- Götz J., Chen F., van Dorpe J. & Nitsch R. M. Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Aβ42 Fibrils. Science 293, 1491–1495 (2001). - PubMed
-
- Lewis J. et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
