This is a preprint.
Inhibitory and disinhibitory VIP IN-mediated circuits in neocortex
- PMID: 40060562
- PMCID: PMC11888407
- DOI: 10.1101/2025.02.26.640383
Inhibitory and disinhibitory VIP IN-mediated circuits in neocortex
Abstract
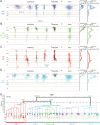
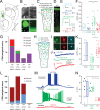
Cortical GABAergic interneurons (INs) expressing the neuropeptide vasoactive-intestinal peptide (VIP) predominantly function by inhibiting dendritic-targeting somatostatin (SST) expressing INs, thereby disinhibiting pyramidal cells (PCs) and facilitating cortical circuit plasticity. VIP INs are a molecularly heterogeneous group, but the physiological significance of this diversity is unclear at present. Here, we have characterized the functional diversity of VIP INs in the primary somatosensory cortex (vS1) using intersectional genetic approaches. We found that VIP INs are comprised of four primary populations that exhibit different laminar distributions, axonal and dendritic arbors, intrinsic electrophysiological properties, and efferent connectivity. Furthermore, we observe that these populations are differentially activated by long-range inputs, and display distinct responses to neuromodulation by endocannabinoids, acetylcholine and noradrenaline. Stimulation of VIP IN subpopulations in vivo results in differential effects on the cortical network, thus providing evidence for specialized modes of VIP IN-mediated regulation of PC activity during cortical information processing.
Keywords: GABAergic microcircuits; VIP interneurons; cortical disinhibitory circuits; endocannabinoid signaling; interneuron diversity; intersectional genetic targeting; neocortical synaptic connectivity; neuromodulatory control; optogenetic manipulation; somatosensory cortex.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Ramón y Cajal S. (1911). Histologie du système nerveux de l’homme et des vertébrés, Édition française revue et mise à jour par l’auteur, traduite de l’espagnol par L. Azoulay Edition (A. Maloine).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
