This is a preprint.
Autophagy Suppresses CCL2 to Preserve Appetite and Prevent Lethal Cachexia
- PMID: 40060612
- PMCID: PMC11888218
- DOI: 10.1101/2025.02.20.638910
Autophagy Suppresses CCL2 to Preserve Appetite and Prevent Lethal Cachexia
Abstract
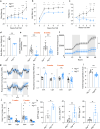
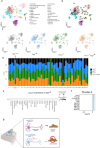
Macroautophagy (autophagy hereafter) captures intracellular components and delivers them to lysosomes for degradation and recycling1. In adult mice, autophagy sustains metabolism to prevent wasting by cachexia and to survive fasting, and also suppresses inflammation, liver steatosis, neurodegeneration, and lethality2,3. Defects in autophagy contribute to metabolic, inflammatory and degenerative diseases, however, the specific mechanisms involved were unclear 4. Here we profiled metabolism and inflammation in adult mice with conditional, whole-body deficiency in an essential autophagy gene and found that autophagy deficiency altered fuel usage, and reduced ambulatory activity, energy expenditure, and food intake, and elevated circulating GDF15, CXCL10, and CCL2. While deletion of Gdf15 or Cxcl10 provided no or mild benefit, deletion of Ccl2 restored food intake, suppressed cachexia and rescued lethality of autophagy-deficient mice. To test if appetite suppression by CCL2 was responsible for lethal cachexia we performed single nucleus RNA sequencing of the hypothalamus, the center of appetite control in the brain. Notably, we found that autophagy deficiency was specifically toxic to PMCH and HCRT neurons that produce orexigenic neuropeptides that promote food intake, which was rescued by deficiency in CCL2. Finally, the restoration of food intake via leptin deficiency prevented lethal cachexia in autophagy-deficient mice. Our findings demonstrate a novel mechanism where autophagy prevents induction of a cachexia factor, CCL2, which damages neurons that maintain appetite, the destruction of which may be central to degenerative wasting conditions.
Figures



References
-
- Karsli-Uzunbas G. et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 4, 914–927 (2014). 10.1158/2159-8290.CD-14-0363 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
