Photobiomodulation improves functional recovery after mild traumatic brain injury
- PMID: 40060762
- PMCID: PMC11883100
- DOI: 10.1002/btm2.10727
Photobiomodulation improves functional recovery after mild traumatic brain injury
Abstract
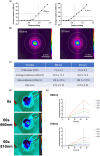
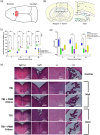
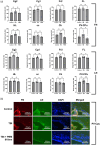
Mild traumatic brain injury (mTBI) is a common consequence of head injury but there are no recognized interventions to promote recovery of the brain. We previously showed that photobiomodulation (PBM) significantly reduced the number of apoptotic cells in adult rat hippocampal organotypic slice cultures. In this study, we first optimized PBM delivery parameters for use in mTBI, conducting cadaveric studies to calibrate 660 and 810 nm lasers for transcutaneous delivery of PBM to the cortical surface. We then used an in vivo weight drop mTBI model in adult rats and delivered daily optimized doses of 660, 810 nm, or combined 660/810 nm PBM. Functional recovery was assessed using novel object recognition (NOR) and beam balance tests, whilst histology and immunohistochemistry were used to assess the mTBI neuropathology. We found that PBM at 810, 660 nm, or 810/660 nm all significantly improved both NOR and beam balance performance, with 810 nm PBM having the greatest effects. Histology demonstrated no overt structural damage in the brain after mTBI, however, immunohistochemistry using brain sections showed significantly reduced activation of both CD11b+ microglia and glial fibrillary acidic protein (GFAP)+ astrocytes at 3 days post-injury. Significantly reduced cortical localization of the apoptosis marker, cleaved caspase-3, and modest reductions in extracellular matrix deposition after PBM treatment, limited to choroid plexus and periventricular areas were also observed. Our results demonstrate that 810 nm PBM optimally improved functional outcomes after mTBI, reduced markers associated with apoptosis and astrocyte/microglial activation, and thus may be useful as a potential regenerative therapy.
Keywords: functional recovery; medical devices; neuroprotection; photobiomodulation; traumatic brain injury.
© 2024 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
Members of the authorship have submitted a patent pending application (DD, MH, WP, AS, and ZA) relating to the invasive delivery of PBM (UK Patent App. No. 2006201.4; US Patent App. 17/922, 157, 2023). There are no other competing interests to declare, including those relating to employment, consultancy, other patents, or products in development.
Figures




References
-
- Maas AIR, Menon DK, David Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987‐1048. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

