Anticancer Activities of Natural and Synthetic Steroids: A Review
- PMID: 40060836
- PMCID: PMC11886665
- DOI: 10.1021/acsomega.4c08577
Anticancer Activities of Natural and Synthetic Steroids: A Review
Abstract
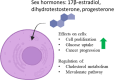
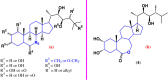
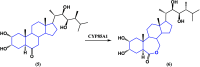
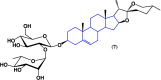
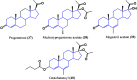
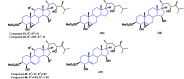
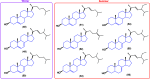
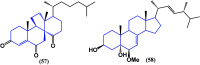
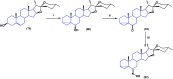
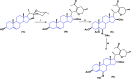
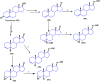
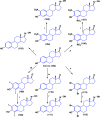
Steroids have demonstrated a wide field of research on the subject of anticancer compounds, particularly antiproliferative with cell lines, with special emphasis on the historical link between steroids and cancer and the use of in silico technologies to understand the impact of natural and synthetic steroids on cancer cells focused on finding common denominators of the type of structural changes that give antiproliferative and/or cytotoxic properties, both in control and cancer cell lines. Through this review and classification by origin and/or synthesis, it is found that steroidal saponins are highly cytotoxic, although with low selectivity against control cells, while on the part of the aglycone the presence of heteroatoms such as nitrogen and oxygen increases the antiproliferative activity, mainly via cell cycle arrest and the induction of apoptosis, mechanisms that have been partially proven, using semisynthetic derivatives, as well as bioconjugates between saponins and nitrogenous steroids with now a high cytotoxicity and selectivity against control cell lines. This gives rise to the idea that steroids as a study model for the design of anticancer agents are an excellent template with a wide field of study.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



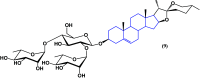
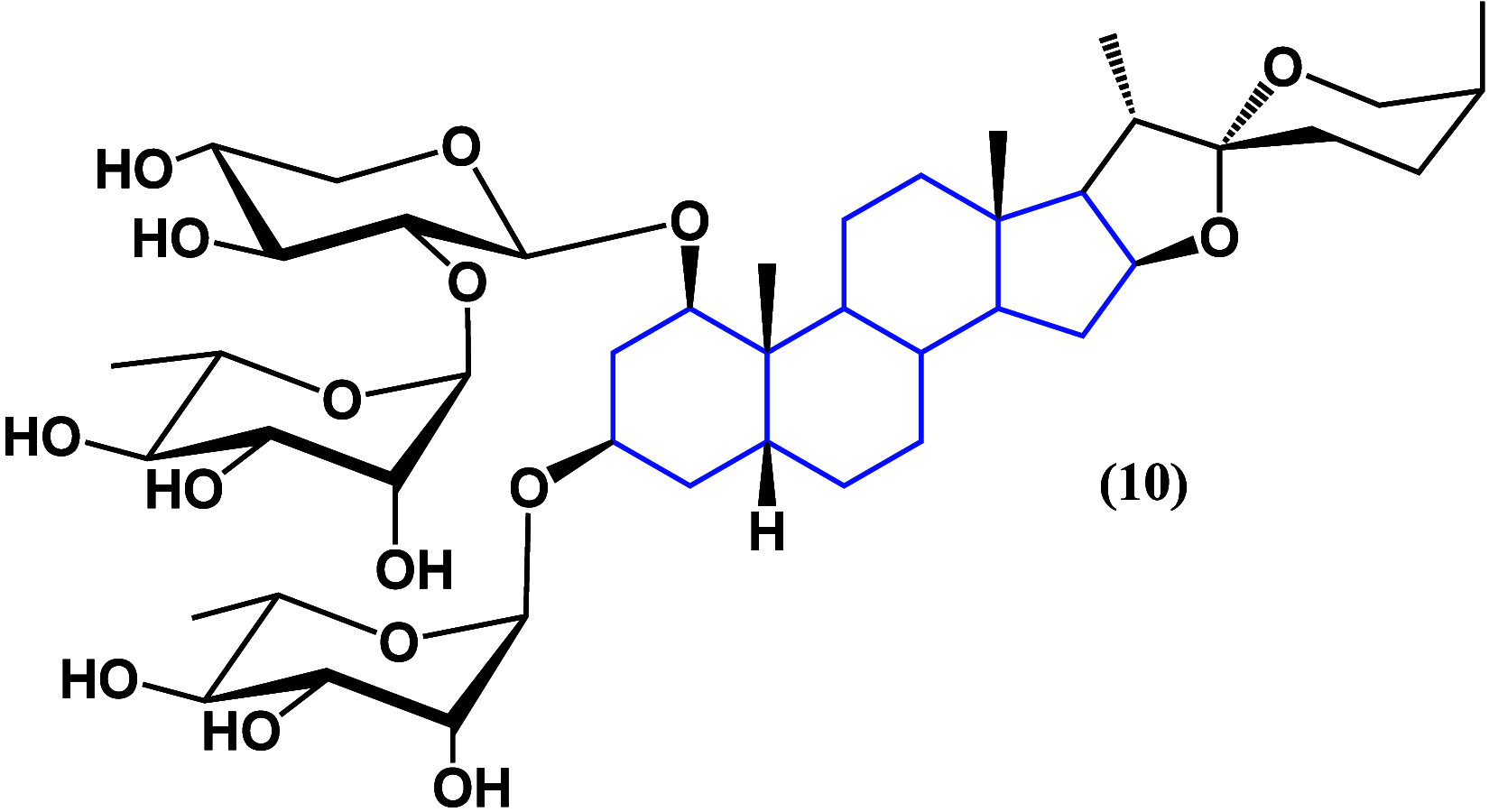

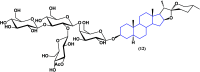
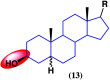
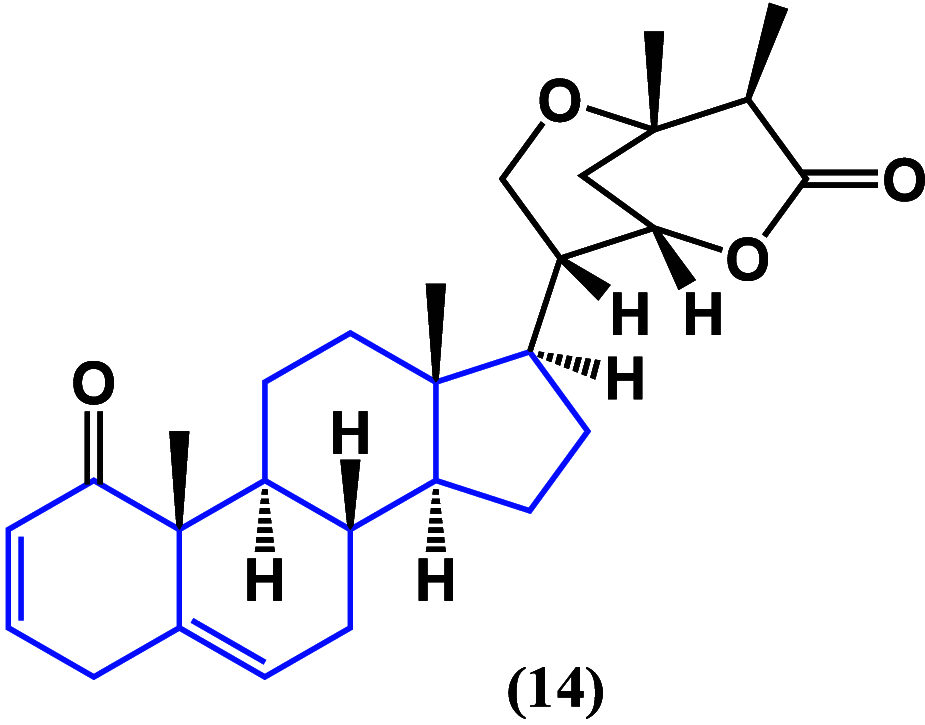
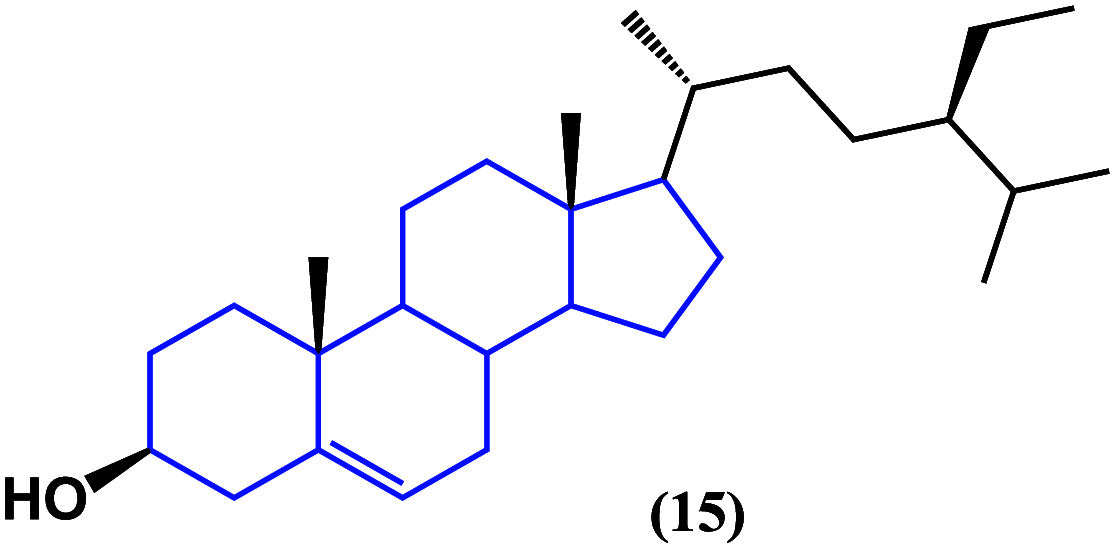
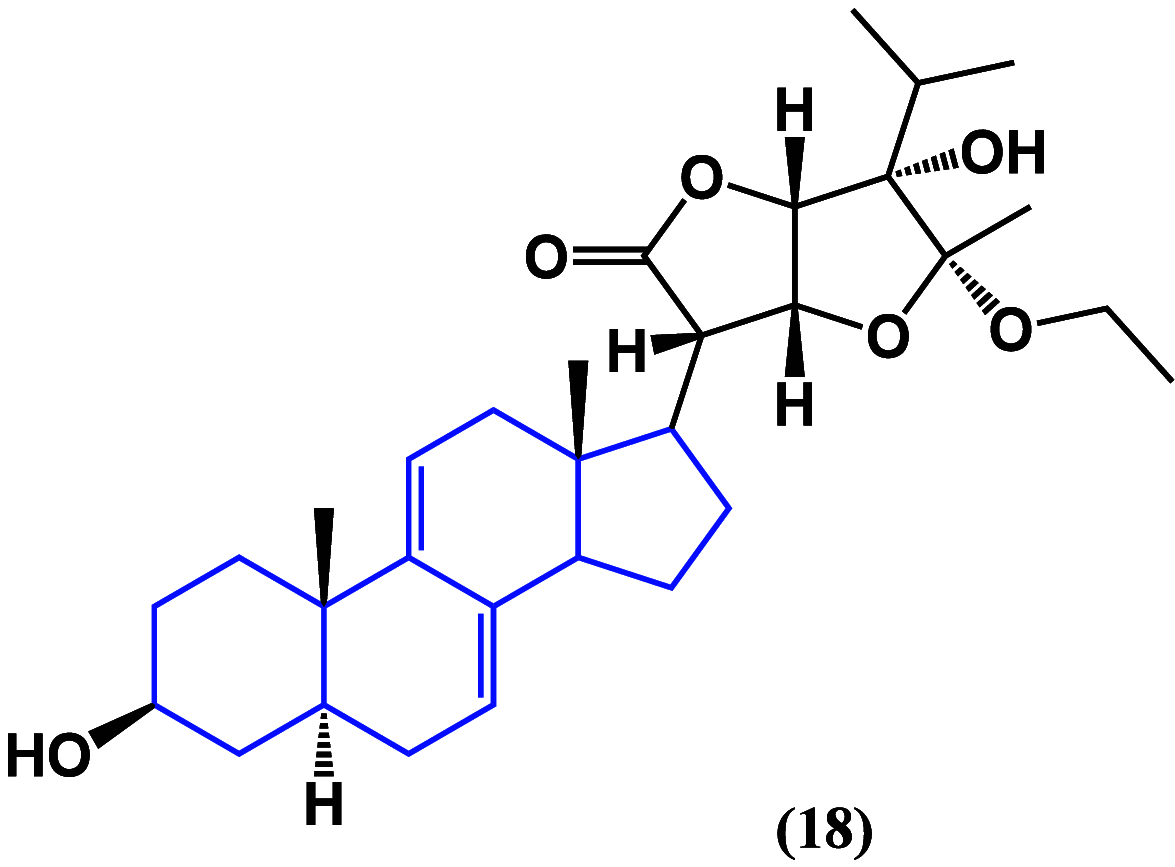
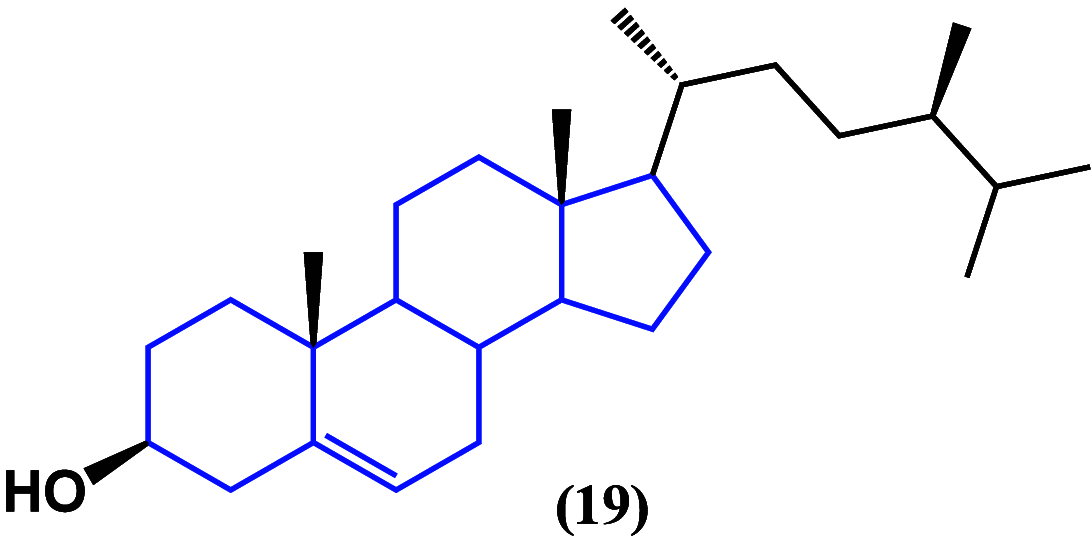
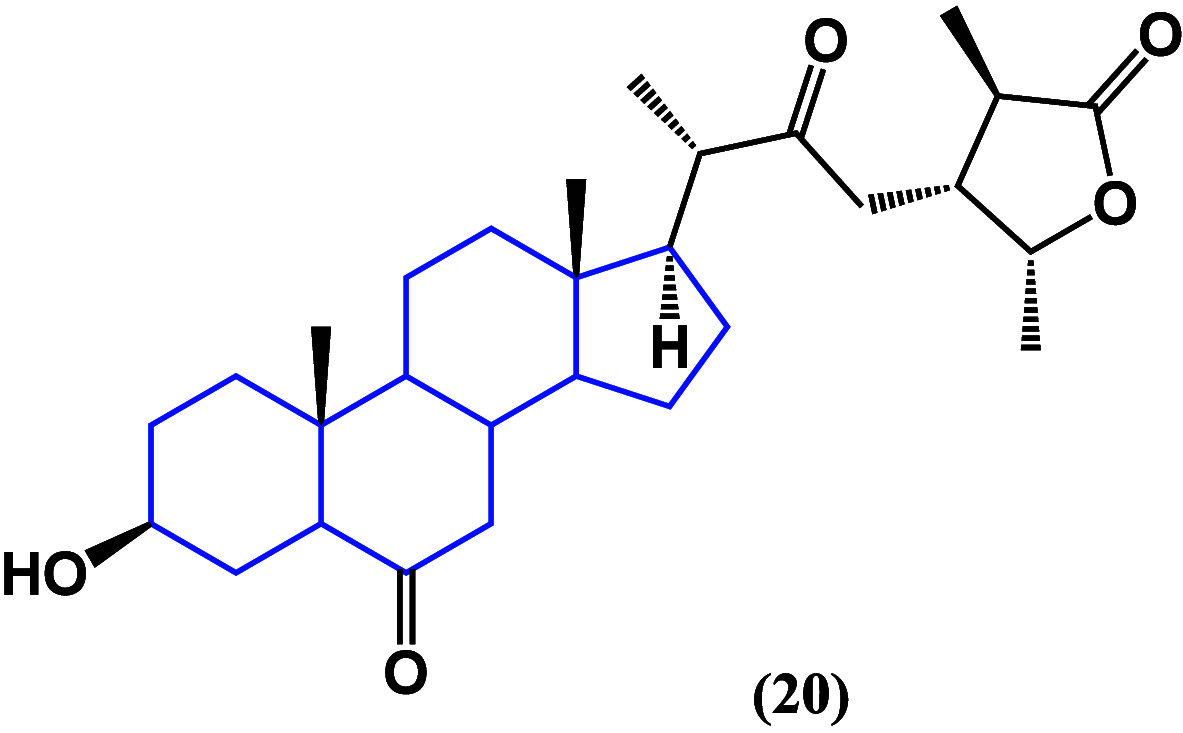
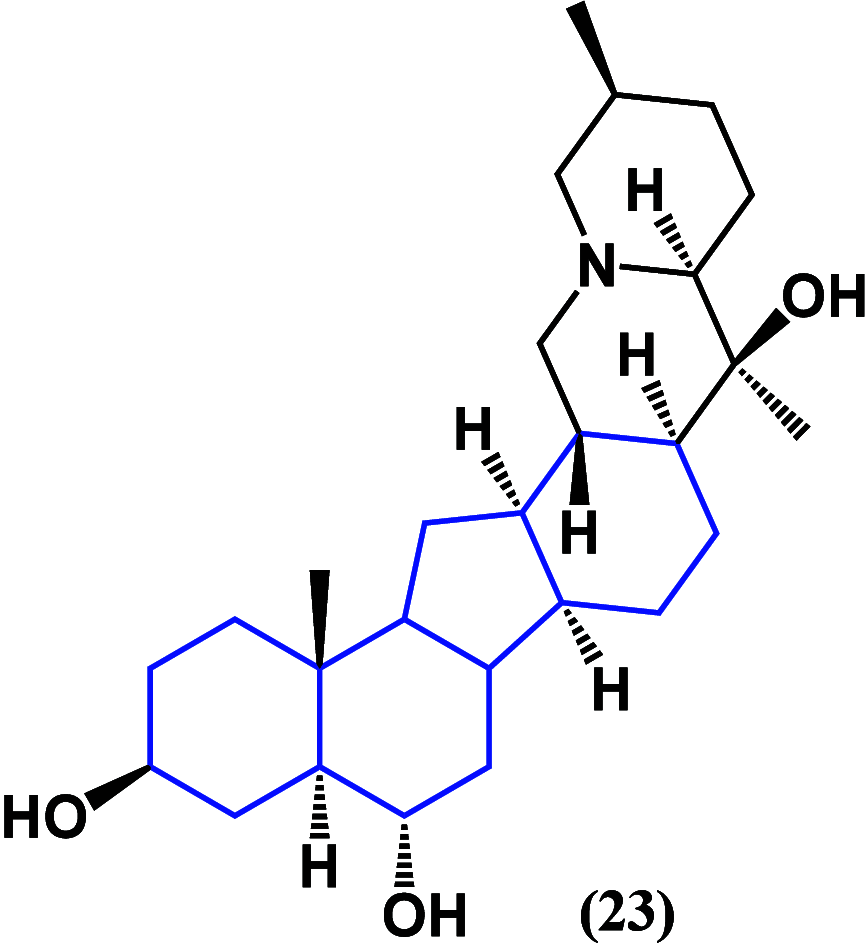
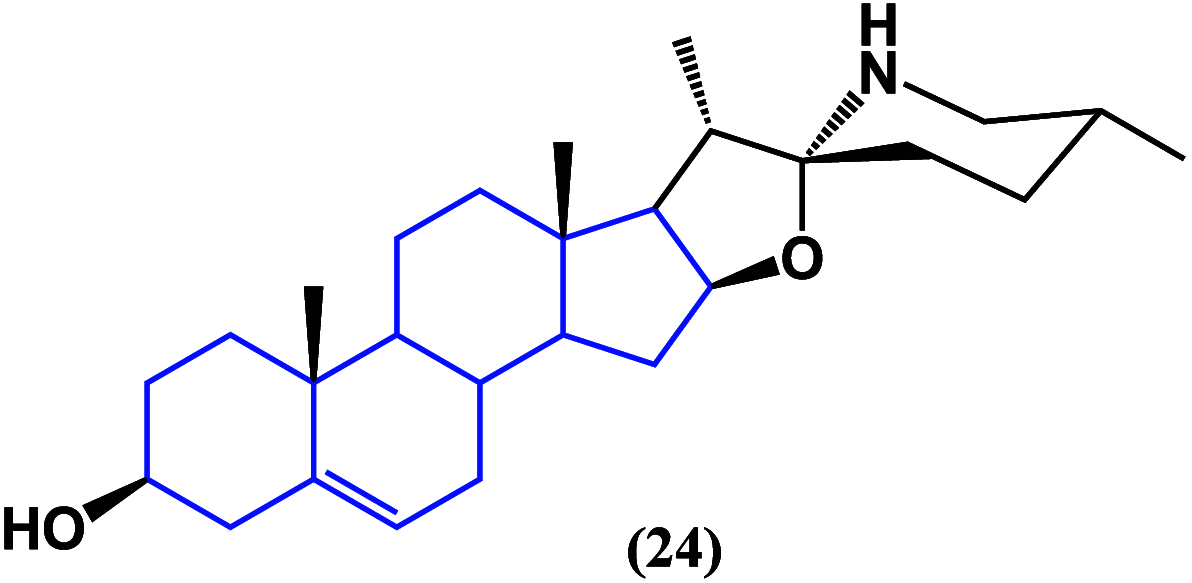
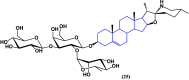
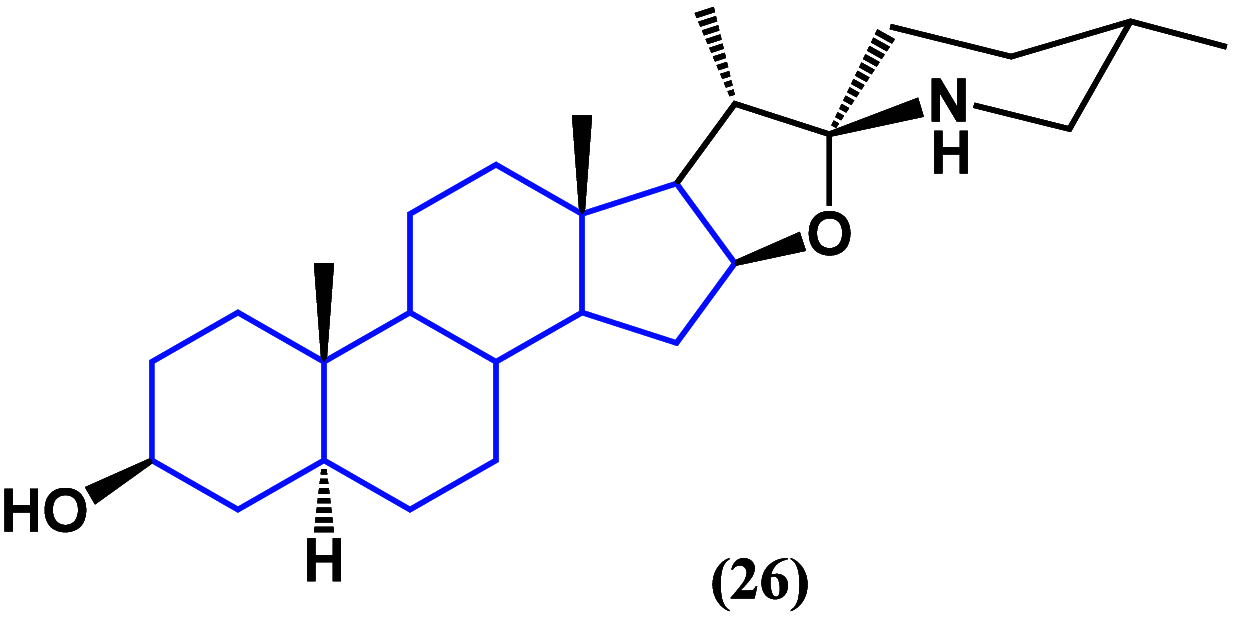

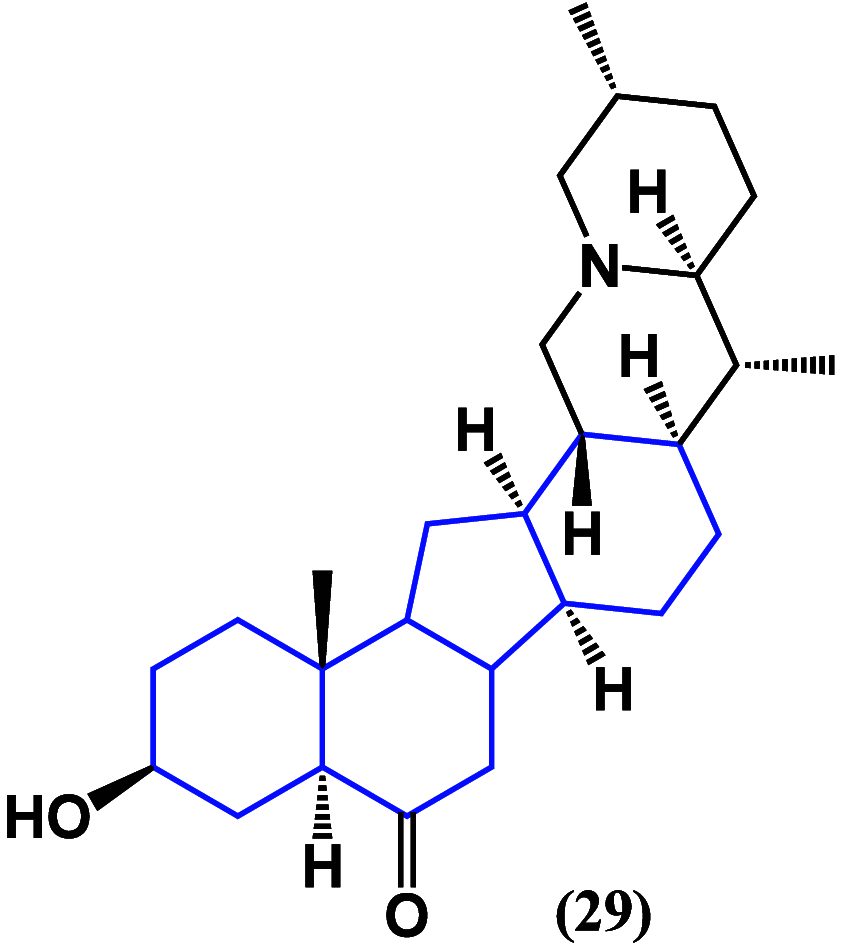




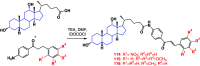
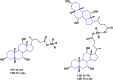
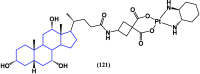
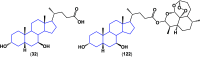
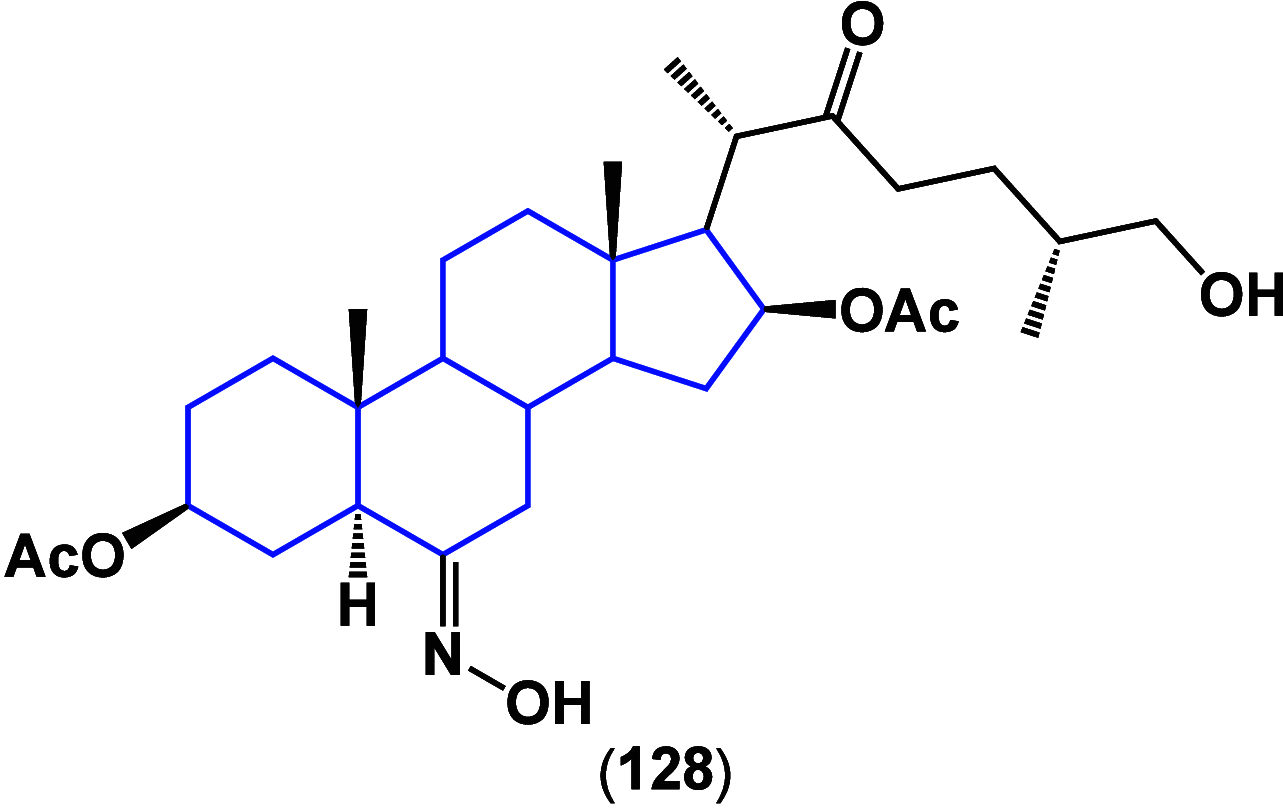
References
-
- Nagorny P.; Cichowicz N.. New Strategy Based on Sequential Michael/Aldol Reactions for the Asymmetric Synthesis of Cardenolides. Strategies and Tactics in Organic Synthesis; Elsevier, 2016; Vol. 12.
-
- Chen Z.; Zhang Y.; Wen Z.; He Y.; Zhang C.; Zhang G.; Han C.; Li Z. Study on the Applicability of Saturated Hydrocarbon Parameters in the Evaluation of Lacustrine Source Rocks and Oils Based on Thermal Simulation Experiments. Processes 2023, 11, 2187.10.3390/pr11072187. - DOI
Publication types
LinkOut - more resources
Full Text Sources
