Papain-Catalyzed Hydrolysis of N α-Benzoyl-arginine- p-nitroanilide in an Aqueous-Organic Medium
- PMID: 40060855
- PMCID: PMC11886738
- DOI: 10.1021/acsomega.4c11059
Papain-Catalyzed Hydrolysis of N α-Benzoyl-arginine- p-nitroanilide in an Aqueous-Organic Medium
Abstract
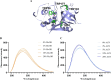
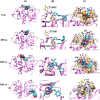
Enzyme-based biosensors have emerged as an effective alternative, providing simplicity, high sensitivity, and the capability to detect multiple residues. However, despite their widespread use, limited studies have examined how organic solvents inhibit these sensors. This study investigates the enzymatic reactions and structure of the selected model enzyme, papain, a protease derived from Carica papaya, in the presence of various organic solvents. Enzyme activity was monitored through the hydrolysis of Nα-benzoyl-arginine-p-nitroanilide (BAPNA), with the resulting yellow product, p-nitroaniline, measured at a wavelength of 430 nm. The experiments incorporated a 10% (v/v) concentration of dimethyl sulfoxide (DMSO) to ensure the solubility of BAPNA. Results showed that methanol and ethanol increased the K m value while causing little change in V max, which negatively impacted the enzyme's catalytic efficiency. In contrast, acetonitrile (ACN) behaved as a reversible mixed-competitive inhibitor of papain, exhibiting lower millimolar IC50 values. Furthermore, an emission maximum shift to lower wavelengths with increasing concentrations of ACN suggested that the tryptophan residues within the enzyme structure were slightly more buried. Molecular dynamics simulations of the BAPNA-papain complex in cosolvent environments containing water, DMSO, and ACN indicated that ACN could act as a mixed-competitive inhibitor alongside BAPNA and that solvent polarity could influence the binding of BAPNA to papain. These findings provide valuable insights for the application of organic solvents in biosensor technologies.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
LinkOut - more resources
Full Text Sources
