Thermally activated delayed fluorescence materials: innovative design and advanced application in biomedicine, catalysis and electronics
- PMID: 40061070
- PMCID: PMC11887472
- DOI: 10.1039/d5ra00157a
Thermally activated delayed fluorescence materials: innovative design and advanced application in biomedicine, catalysis and electronics
Abstract
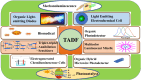
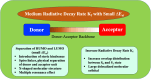
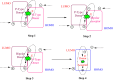
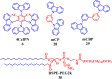
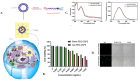
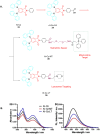
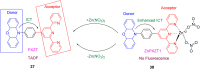
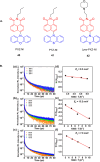
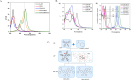
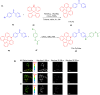
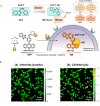
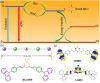
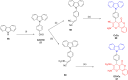
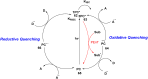
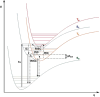
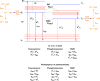
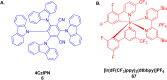
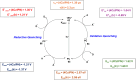
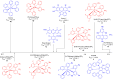
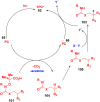
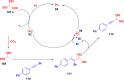
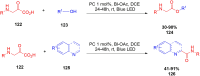
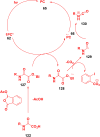
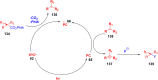
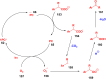
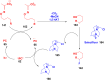
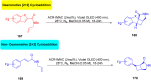
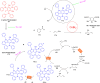
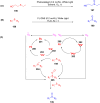
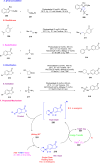
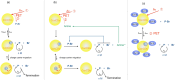
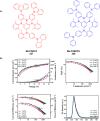
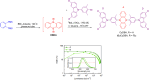
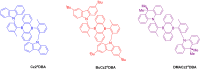
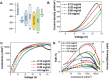
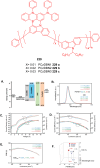
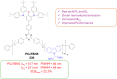
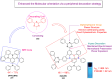
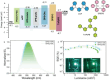
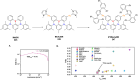
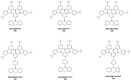
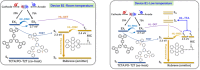
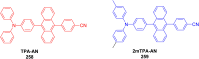
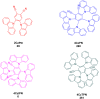
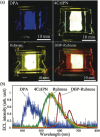
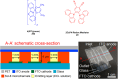
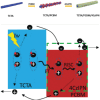
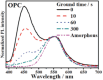
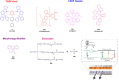
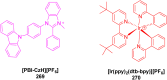
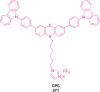
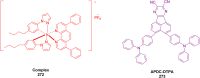
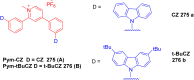
Thermally Activated Delayed Fluorescence (TADF) materials have emerged as a revolutionary class of functional compounds, driven by their unique ability to utilize excitons from both singlet and triplet states for efficient fluorescence emission. This manuscript provides an overview of recent innovations in TADF material design, focusing on molecular strategies to achieve optimal TADF properties, including small singlet-triplet energy gaps (ΔE ST) and high photoluminescence quantum yields. We explore the diverse applications of TADF materials, spanning OLEDs, biomedical imaging, photosensitizers, photocatalysis, UV photodetectors (UVOPDs), electrogenerated chemiluminescence, triplet-triplet annihilation (TTA) sensitizers, organic hybrid microwire radial heterojunctions, multicolor luminescent micelles, mechano-luminescence (ML), light-emitting electrochemical cells (LEECs), and fluorescent probes. The integration of TADF materials in these technologies highlights their potential to enhance performance and efficiency. Through this review, we aim to elucidate the fundamental principles governing TADF behavior and present a forward-looking perspective on the synthetic methodologies and new, versatile applications of materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

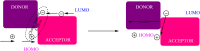

























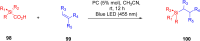
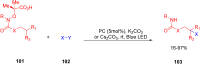
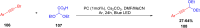

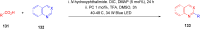




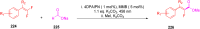

























References
Publication types
LinkOut - more resources
Full Text Sources

