This is a preprint.
Trajectories of microbiome-derived bile acids in early life - insights into the progression to islet autoimmunity
- PMID: 40061321
- PMCID: PMC11888530
- DOI: 10.1101/2025.02.18.25322275
Trajectories of microbiome-derived bile acids in early life - insights into the progression to islet autoimmunity
Abstract
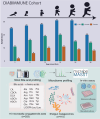
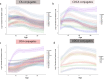
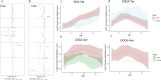
Recent studies reveal that gut microbes produce diverse bile acid conjugates, termed microbially conjugated bile acids (MCBAs). However, their regulation and health effects remain unclear. Here, we analyzed early-life MCBA patterns and their link to islet autoimmunity. We quantified 110 MCBAs in 303 stool samples collected longitudinally (3-36 months) from children who developed one or more islet autoantibodies and controls who remained autoantibody-negative. Stool MCBAs showed distinct age-dependent trajectories and correlated with gut microbiome composition. Altered levels of ursodeoxycholic and deoxycholic acid conjugates were linked to islet autoimmunity as well as modulated monocyte activation in response to immunostimulatory lipopolysaccharide and Th17/Treg cell balance. These findings suggest MCBAs influence immune development and type 1 diabetes risk.
Conflict of interest statement
Competing interests: P.C.D. is an advisor and holds equity in Cybele and Sirenas, is a science advisor and holds equity in bileOmix and is a Scientific co-founder, advisor and holds equity to Ometa, Enveda, and Arome with prior approval by UC-San Diego. PCD also consulted for DSM animal health in 2023. The other authors declare no competing interests.
Figures



References
-
- Hofmann A. F. et al. A proposed nomenclature for bile acids. Journal of lipid research 33, 599–604 (1992). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
