This is a preprint.
Genetic subtyping of obesity reveals biological insights into the uncoupling of adiposity from its cardiometabolic comorbidities
- PMID: 40061343
- PMCID: PMC11888528
- DOI: 10.1101/2025.02.25.25322830
Genetic subtyping of obesity reveals biological insights into the uncoupling of adiposity from its cardiometabolic comorbidities
Update in
-
Genetic subtyping of obesity reveals biological insights into the uncoupling of adiposity from its cardiometabolic comorbidities.Nat Med. 2025 Sep 12. doi: 10.1038/s41591-025-03931-0. Online ahead of print. Nat Med. 2025. PMID: 40940440
Abstract
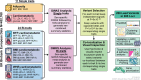
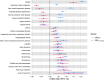
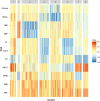
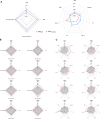
Obesity is a highly heterogeneous disease that cannot be captured by one single adiposity trait. Here, we performed a multi-trait analysis to study obesity in the context of its common cardiometabolic comorbidities, acknowledging that not all individuals with obesity suffer from cardiometabolic comorbidities and that not all those with normal weight clinically present without them. We leveraged individual-level genotype-phenotype data of 452,768 individuals from the UK Biobank and designed uncoupling phenotypes that are continuous and range from high adiposity with a healthy cardiometabolic profile to low adiposity with an unhealthy cardiometabolic profile. Genome-wide association analyses of these uncoupling phenotypes identified 266 independent variants across 205 genomic loci where the adiposity-increasing allele is also associated with a lower cardiometabolic risk. Consistent with the individual variant effects, a genetic score (GRSuncoupling) that aggregates the uncoupling effects of the 266 variants was associated with lower risk of cardiometabolic disorders, including dyslipidemias (OR=0.92, P=1.4×10-89), type 2 diabetes (OR=0.94, P=6×10-21), and ischemic heart disease (OR=0.96, P=7×10-11), despite a higher risk of obesity (OR=1.16, P=4×10-108), which is in sharp contrast to the association profile observed for the adiposity score (GRSBFP). Nevertheless, a higher GRSuncoupling score was also associated with a higher risk of other, mostly weight-bearing disorders, to the same extent as the GRSBFP. The 266 variants clustered into eight subsets, each representing a genetic subtype of obesity with a distinct cardiometabolic risk profile, characterized by specific underlying pathways. Association of GRSuncoupling and GRSBFP with levels of 2,920 proteins in plasma found 208 proteins to be associated with both scores. The majority (85%) of these overlapping GRS-protein associations were directionally consistent, suggesting adiposity-driven effects. In contrast, levels of 32 (15%) proteins (e.g. IGFBP1, IGFBP2, LDLR, SHBG, MSTN) had opposite directional effects between GRSBFP and GRSuncoupling, suggesting that cardiometabolic health, and not adiposity, associated with their levels. Follow-up analyses provide further support for adipose tissue expandability, insulin secretion and beta-cell function, beiging of white adipose tissue, inflammation and fibrosis. They also highlight mechanisms not previously implicated in uncoupling, such as hepatic lipid accumulation, hepatic control of glucose homeostasis, and skeletal muscle growth and function. Taken together, our findings contribute new insights into the mechanisms that uncouple adiposity from its cardiometabolic comorbidities and illuminate some of the heterogeneity of obesity, which is critical for advancing precision medicine.
Figures


References
-
- Mokdad A.H., et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003). - PubMed
-
- Alberti K.G., et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009). - PubMed
-
- Hubert H.B., Feinleib M., McNamara P.M. & Castelli W.P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67, 968–977 (1983). - PubMed
-
- Must A., et al. The disease burden associated with overweight and obesity. JAMA 282, 1523–1529 (1999). - PubMed
Publication types
Grants and funding
- U01 HG007417/HG/NHGRI NIH HHS/United States
- R01 HL158884/HL/NHLBI NIH HHS/United States
- 75N92022D00002/HL/NHLBI NIH HHS/United States
- R01 DK107786/DK/NIDDK NIH HHS/United States
- R01 DK075787/DK/NIDDK NIH HHS/United States
- S10 OD026880/OD/NIH HHS/United States
- R01 DK123019/DK/NIDDK NIH HHS/United States
- R01 HL151152/HL/NHLBI NIH HHS/United States
- 75N92022D00003/HL/NHLBI NIH HHS/United States
- UM1 DK126185/DK/NIDDK NIH HHS/United States
- R01 HL142302/HL/NHLBI NIH HHS/United States
- R01 DK110113/DK/NIDDK NIH HHS/United States
- 75N92022D00004/HL/NHLBI NIH HHS/United States
- R01 DK102173/DK/NIDDK NIH HHS/United States
- U01 HG011723/HG/NHGRI NIH HHS/United States
- R01 HL156991/HL/NHLBI NIH HHS/United States
- UL1 TR004419/TR/NCATS NIH HHS/United States
- S10 OD030463/OD/NIH HHS/United States
- 75N92022D00005/HL/NHLBI NIH HHS/United States
- R01 HG010297/HG/NHGRI NIH HHS/United States
- 75N92022D00001/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
